| Child Kidney Dis > Volume 27(2); 2023 > Article |
|
Abstract
Here, we present the case of a 2-month-old male infant with hyponatremic hypertensive syndrome resulting from stenosis of the right proximal and mid-renal arteries. The patient exhibited nephrotic-range proteinuria, low serum albumin, increased serum creatinine, and elevated renin and aldosterone levels. Doppler ultrasonography and computed tomography angiography revealed decreased vascular flow in the small right renal artery. Following a successful percutaneous balloon angioplasty, the patient experienced a decrease in blood pressure and normalization of serum electrolyte levels within a few days. However, it took 3 months for the proteinuria to resolve completely. This case is significant as it represents the first reported instance of a neonate presenting with clinical features resembling congenital nephrotic syndrome caused by renal artery stenosis that was successfully treated with percutaneous renal angioplasty.
Hyponatremic hypertensive syndrome (HHS) is characterized by hypertension, hyponatremia, and hypokalemia, which are attributed to unilateral stenosis or occlusion of the renal artery [1]. Approximately 16% of adults with unilateral renal artery stenosis have been reported to suffer from HHS [2]. The prevalence of HHS in children is not well known, but it is considered a rare disease [3,4]. However, a previous study reported that HHS was observed in 28% of pediatric patients with renovascular hypertension in a single center [2]. Patients with HHS may develop central nervous system symptoms such as convulsions, altered mental status due to hypertension, and combined hyponatremia [3,5]. Patients may also experience polyuria, polydipsia, and nephrotic-range proteinuria [2]. In most cases, the disease can be managed by addressing the underlying cause, controlling blood pressure, and providing supportive care for dehydration and hyponatremia [1]. Herein, we report a case of HHS in a 2-month-old infant who presented with nephrotic syndrome and improved after percutaneous balloon angioplasty.
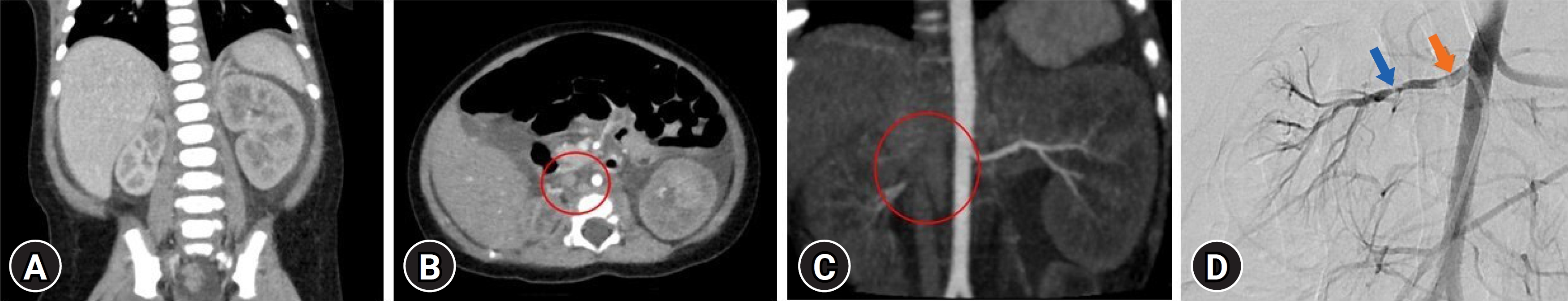
A 2-month-old male infant with no specific birth history presented to the hospital with general weakness, vomiting, and poor oral intake for 5 days. At the time of admission, the patientŌĆÖs initial body weight was 6.6 kg, which was 400 g lower than usual, and mottled skin was observed. His blood pressure was severely elevated, measuring 143/107 mmHg. Laboratory tests revealed proteinuria in the nephrotic range, with a urine protein-to-creatinine ratio of 107 mg/mg, as well as a low serum albumin level of 2.6 g/dL, low serum sodium level of 123 mmol/L, increased serum creatinine level of 0.8 mg/dL, and elevated renin and aldosterone levels of >80 ng/ml/hr (0ŌĆō3 years reference range: <16.6 ng/mL/hr) and 206 ng/dL (1ŌĆō11 months reference range: 6.5ŌĆō86.0 ng/dL). The patient developed atonic seizures and perioral cyanosis on the second day after admission. The patient received intravascular anticonvulsants, antihypertensive medications, and a 3% saline solution. Kidney Doppler ultrasonography revealed hypotrophy of the right kidney, decreased vascular flow due to narrowing of the right renal artery, and compensatory hypertrophy of the left kidney. Brain magnetic resonance imaging, electroencephalogram and tests for viral infections (toxoplasmosis, rubella, cytomegalovirus, herpes, human immunodeficiency virus, and syphilis) performed on this patient were all negative, and the next-generation sequencing panel for diagnosing podocytopathy-related gene mutations was negative. The patient had no significant family history related to thrombosis or stenosis. The patient underwent renal computed tomography angiography, and the results showed that the left renal artery was intact; however, the proximal ostium of the right renal artery was not clearly visible, and there was a suspected small right renal artery (Fig. 1A-C). The patient underwent percutaneous abdominal aortography and balloon angioplasty to improve right renal artery stenosis. Aortography revealed several focal stenoses in the right proximal and mid-renal arteries, suggesting thrombi accompanied by a focal filling defect (Fig. 1D). Through balloon angioplasty, the blood flow was improved by treating the stenosis. After the procedure, the patient's general condition improved, blood pressure decreased, and antihypertensive medication was tapered. After the procedure, serum albumin, sodium, renin, aldosterone, and creatinine levels were restored to normal. Proteinuria decreased after treatment, but nephrotic-range proteinuria persisted. There were no abnormal findings on antithrombin, protein C, protein S, antiphospholipid antibody, or factor 5, 7, 8, or 10 tests for detecting thrombophilia. Administration of low-molecular-weight heparin was initiated to manage the patient's residual renal artery thrombi, which was then switched to warfarin. Nineteen days after the procedure, the patient was discharged with warfarin and antihypertensive drugs. The proteinuria disappeared after 3 months without antiproteinuric medication. Six months later, a follow-up renal computed tomography angioplasty confirmed that the residual thrombosis had disappeared; therefore, warfarin was discontinued.
Our case represents the first reported case of a neonate presenting with massive proteinuria, hypoalbuminemia, and hypertension caused by renal artery stenosis that was successfully treated with percutaneous renal angioplasty. These clinical features should be differentially diagnosed from congenital nephrotic syndrome.
Congenital nephrotic syndrome is a heterogeneous group of disorders characterized by nephrotic-range proteinuria and hypoalbuminemia, which typically manifests in infants younger than 3 months. It is a rare disease classified into primary congenital nephrotic syndrome, caused by genetic defects in podocyte proteins such as nephrin and podocin, and secondary congenital nephrotic syndrome, caused by infections (e.g., syphilis, toxoplasmosis, cytomegalovirus, rubella, hepatitis B, and human immunodeficiency virus) or maternal alloimmune disease (e.g., maternal systemic lupus erythematosus) [6]. Several case reports have demonstrated that HHS resulting from renal artery stenosis and thrombosis can lead to massive proteinuria and/or hypoalbuminemia [2,5,7,8]. Neonatal cases of nephrotic syndrome and HHS caused by renal arterial stenosis are rare. When ischemia occurs in the unilateral kidney, overstimulation of the renin-angiotensin-aldosterone system in the ischemic kidney causes hypertension in HHS. Additionally, hyperfiltration in the contralateral kidney leads to secondary polyuria, renal electrolyte loss, including natriuresis, and hypovolemia. The pathophysiology of massive proteinuria in HHS has not been clearly elucidated. Some theories include ultrafiltration, focal segmental glomerular sclerosis development, and podocyte injury associated with angiotensin II [2,9,10]. Therefore, patients with renal artery stenosis should be considered HHS due to renal artery hypertension. Renovascular hypertension in children is one of the most common causes of secondary hypertension. In previous studies, HHS was confirmed in about 1/4 of renovascular hypertension [2], so we should be careful not to underdiagnose HHS by reviewing the patient's clinical features and laboratory findings.
Patients diagnosed with HHS typically undergo treatment with antihypertensive medication and supportive care and may require invasive procedures and surgery to alleviate symptoms. Previously reported cases of neonates with HHS have been managed with medication and/or nephrectomy [4,5,11,12]. In our case, the patient underwent successful percutaneous balloon angioplasty, which preserved the function of the affected kidney. To our knowledge, this is the first report on using percutaneous balloon angioplasty to treat HHS in neonates. Percutaneous balloon angioplasty is an effective intervention for improving hypertension and correcting electrolyte imbalance in patients with HHS [2,8,13,14]. Angioplasty is considered the optimal choice for preserving kidney function in patients with renal artery stenosis, especially in children with a longer lifespan.
In our patient, multiple thrombi with renal artery stenosis were observed. The occurrence of thrombosis in neonates is rare. A German registry reported the incidence of symptomatic renal vein thrombosis in neonates to be at least 2.2 per 100,000 live births and renal artery stenosis is even more uncommon. This can be related to factors such as indwelling catheter insertion, for example, umbilical artery catheterization as well as disseminated intravascular coagulation, impaired liver function, fluctuations in cardiac output, and congenital heart diseases in neonates [5,15]. Our patient had no history of thrombosis, and thrombophilic conditions were excluded based on laboratory tests. However, it remains unclear whether renal artery thrombosis results in renal artery stenosis or whether hypoplastic kidney itself affects artery stenosis. However, renal artery stenosis and nephrotic syndrome can also cause thrombosis. Monitoring for additional thrombotic events is necessary in the future. At the last follow-up at 3 years of age, the patient did not experience any other thrombotic events.
Our patient presented with neurological symptoms at treatment initiation. HHS induces secondary hyperreninemia and hyperaldosteronism, further exacerbating salt and water loss and leading to malignant hypertension [2,4,13]. This condition has been associated with progressive target organ damage in children, including encephalopathy and intracranial hemorrhage. Central nervous system symptoms occur in approximately half of patients with HHS and are caused by encephalopathy resulting from severe hypertension and hyponatremia [2,3,5,7,8,12]. While most patients recover when electrolyte imbalance and hypertension improve, some patients may still have residual central nervous system sequelae [5,7]. Therefore, early detection and timely treatment of HHS are crucial for preventing neurological sequelae.
In our case, the presence of HHS provided clues regarding the underlying condition. Early diagnosis and appropriate management of HHS are essential to mitigate the risk of complications associated with persistent malignant hypertension and electrolyte imbalance in neonates.
Notes
Ethical statements
This case was reviewed and approved by the Institutional Review Board of Seoul National University Hospital and a waiver the requirement to obtain any informed consent (IRB No. H-2306-190-1444).
Conflicts of interest
Hee Gyung Kang and Ji Hyun Kim are editorial board member of the journal but were not involved in the peer reviewer selection, evaluation, or decision process of this article. No other potential conflicts of interest relevant to this article were reported.
Author contributions
Conceptualization: HGK
Data curation: DK, SHL
Investigation: DK
Methodology: YHA, HGK, JHK, SHL
Project administration: YHY, HGK, JHK, SHL
Visualization: YHA, HGK, JHK
Writing-original draft: DK
Writing-review & editing: YHA, SHL
All authors have read and approved the final manuscript.
References
2. Kovalski Y, Cleper R, Krause I, Dekel B, Belenky A, Davidovits M. Hyponatremic hypertensive syndrome in pediatric patients: is it really so rare? Pediatr Nephrol 2012;27:1037-40.



3. Dixit MP, Hughes JD, Theodorou A, Dixit NM. Hyponatremic hypertensive syndrome (HHS) in an 18-month old-child presenting as malignant hypertension: a case report. BMC Nephrol 2004;5:5.


4. Oliveira JC, Martins MM, Martins E, Rocha G, Moura C, Pinto H, et al. Hyponatremic hypertensive syndrome secondary to renal ischemia: case report. J Pediatr Neonatal Individualized Med 2018;7:e070110.

5. van Tellingen V, Lilien M, Bruinenberg J, de Vries WB. The hyponatremic hypertensive syndrome in a preterm infant: a case of severe hyponatremia with neurological sequels. Int J Nephrol 2011;2011:406515.



6. Boyer O, Schaefer F, Haffner D, Bockenhauer D, Holtta T, Berody S, et al. Management of congenital nephrotic syndrome: consensus recommendations of the ERKNet-ESPN Working Group. Nat Rev Nephrol 2021;17:277-89.




7. Peco-Antic A, Dimitrijevic N, Jovanovic O, Marsenic O, Kostic M. Hyponatremic hypertensive syndrome. Pediatr Nephrol 2000;15:286-9.


8. Mukherjee D, Sinha R, Akhtar MS, Saha AS. Hyponatremic hypertensive syndrome: a retrospective cohort study. World J Nephrol 2017;6:41-4.

9. Schiefer J, Chatzikyrkou C, Mertens PR, Liakopoulos V. Remission of nephrotic syndrome after resolution of renal artery stenosis in a patient with a single functional kidney. Clin Nephrol 2019;91:265-7.


10. Bhardwaj R, Dosani I, Clark BA. Steroid-responsive nephrotic syndrome and bilateral renal artery stenosis: a possible role for angiotensin-mediated podocyte injury. Case Rep Nephrol Urol 2012;2:59-64.



11. Blanc F, Bensman A, Baudon JJ. Renovascular hypertension: a rare cause of neonatal salt loss. Pediatr Nephrol 1991;5:304-6.



12. Daftary AS, Patole SK, Whitehall J. Hypertension-hyponatremia syndrome in neonates: case report and review of literature. Am J Perinatol 1999;16:385-9.


13. Seracini D, Pela I, Favilli S, Bini RM. Hyponatraemic-hypertensive syndrome in a 15-month-old child with renal artery stenosis. Pediatr Nephrol 2006;21:1027-30.



Fig.┬Ā1.
(A) Renal computed tomography (CT) angiography showing asymmetric small size of right kidney with compensatory left renal hypertrophy. (B, C) Renal CT angiography showing the invisible of the right renal artery proximal portion (red circle). (D) Abdomen aortography showing the right renal artery proximal filling defect (red arrow) and mid-portion stenosis (blue arrow).
- TOOLS
-
 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link XML Download
XML Download Full text via DOI
Full text via DOI-
 Download Citation
Download Citation
- Download Citation
-
- Close
 Print
Print-
Share :
-
METRICS

-
- 0 Crossref
- 0 Scopus
- 682 View
- 11 Download
- ORCID iDs
-
Dabin Kim

https://orcid.org/0000-0002-2563-9228Yo Han Ahn

https://orcid.org/0000-0002-8185-4408Hee Gyung Kang

https://orcid.org/0000-0001-8323-5320Ji Hyun Kim

https://orcid.org/0000-0001-8477-0157Seon Hee Lim

https://orcid.org/0000-0001-8327-7002 - Related articles




