Clinical and Pathological Findings of Renal Biopsy in Children: Outcomes from a Single Center Over 27 Years
Article information
Abstract
Purpose
To classify the results of renal biopsy in pediatric patients and to compare pathological findings with clinical features.
Methods
This study included data of 318 children who underwent renal biopsy at our hospital between December 1987 and November 2014. Biopsy specimens were examined histopathologically using light, immunofluorescence, and electron microscopy.
Results
Asymptomatic urinary abnormalities was the most common clinical diagnosis (35.9%), followed by nephrotic syndrome (29.3%), and acute glomerulonephritis (18.0%). Glomerular disease was identified in 98.1% of the renal biopsy specimens. The most common primary cause of glomerulonephritis was IgA nephropathy, with gross hematuria in 61.9% of the patients, hypertension in 14.2%, proteinuria >1.0 gm/24-hr in 33.3%, and impaired renal function in 3.6% patients.
Conclusion
The most common clinical diagnosis was asymptomatic urinary abnormalities, with primary glomerular disease being the most common renal biopsy finding, and IgA nephropathy the most common histopathological lesion. This study provides a 27-year overview of pediatric renal disease at our center and underlines the importance of renal biopsy for accurate diagnosis and proper management.
Introduction
The diagnosis and treatment of pediatric kidney diseases is based on medical history and physical findings. However, in certain cases, a discrepancy between clinical and pathological findings exists, with patients exhibiting similar clinical symptoms despite different underlying pathological features. Although percutaneous renal biopsy is a widely used medical procedure for the diagnosis of kidney disease in adults, the procedure has not been considered suitable for pediatric patients. However, with recent innovations in technique for kidney localization and biopsy, percutaneous renal biopsy is now regarded to be both safe and advisable for the diagnosis of renal disease in pediatric patients and to inform treatment [14]. The indications for renal biopsy vary according to the ethnic and age characteristics of the population studied as well as the geographic location [5].
The aim of our study was to classify results of renal biopsy in pediatric pa tients, based on pathological findings, and to compare the pathological diagnosis to clinical findings.
Material and methods
1. Subjects
A retrospective study of 345 inpatients who underwent renal biopsy in the Pediatric Department of Chonbuk National University Children’s Hospital, between December 1987 and November 2014. The study adhered to the principles of the Declaration of Helsinki and was approved by the Life Science Research Ethic Council Committee of the Chonbuk National University Hospital (CUH 2016-10-009).
Among the 345 patients who underwent biopsy over the study period, 23 were excluded due to incomplete demographic or clinical data, and four due to inappropriate tissue samples (<5 glomeruli in the kidney tissue sample). Prior to renal biopsy, prothrombin and bleeding/clotting time were assessed to determine bleeding tendency. Consent for the procedure was obtained from parents and/or guardians prior to the procedure.
Over the 27-year period of the study, there was considerable variation in the biopsy technique used. Early in the study period, biopsies were performed under sedation using a biopsy gun under fluoroscopy. Since 1997, biopsies were performed under sedation, using an automated gun under real time ultrasound guidance. All biopsies were performed by a pediatric nephrologist. For all biopsies, two cores of kidney tissues were obtained for histopathology assessment, by light microscopy, immunofluorescence microscopy, and electron microscopy (LM, IF, and EM, respectively).
2. Data retrieval
The following clinical and laboratory data were extracted from the clinical records for analysis: patient’s age and sex; serum total protein, albumin, cholesterol, blood urea nitrogen (BUN), creatinine (Cr), and electrolytes; complement 3 (C3) and 4 (C4) and anti-streptolysin-O (ASO) titer; glomerular filtration rate (mL/min/1.73m2=kL/Pcr, k: 0-1 years of age 0.45, 2-12 years of age 0.55, Jaffe method); and 24-hr urine protein (mg/m2/hr).
3. Pathological study
For LM examination, 10 serial sections were cut and prepared using standard procedures and stained with hematoxylin-eosin (HE), Masson’s trichrome, periodic acid-Schiff stain, and silver stains. Tissue specimens for IF examination are snap-frozen in liquid nitrogen and cut on a cryotome. The tissue was stained by the direct method using fluorescein isothiocyanate-conjugated antisera mono specific for immunoglobulin (Ig) G (IgG), IgA, IgM, C3, and fibrinogen. Tissue samples for EM examination were stained on copper 300 mesh grids with uranyl acetate and lead citrate for assessment.
4. Statistical analyses
Data was analyzed using SPSS software (version 22.0; IBM Corporation, Armonk, NY, USA). Simple descriptive statistics, mean and standard deviation (SD), were calculated for continuous variables, namely age, and clinical and laboratory measures. Percentages were calculated for categorical data.
Results
1. Sex and age distribution
Among the 318 cases included in this study, 205 were male (64.5%) and 113 cases female (35.5%), for a male-to-female sex ratio of 1.8:1. The mean±SD age was 9.7±3.6 years (range, 5 months to 18 years), with the following age distribution: 131 cases (41.2%) between 11-15 years of age; 129 (40.5%) between 6-10 years of age; 46 (14%) between 0-5 years of age; and 12 (3.7%) between 16-18 years of age.
2. Indications for renal biopsy
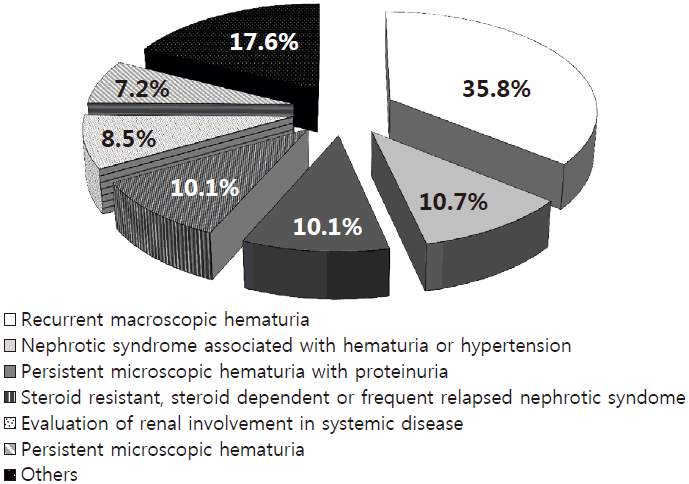
The indications for biopsy were as follows (Fig. 1): Recurrent macroscopic hematuria in 114 cases (35.8%); nephrotic syndrome, with hematuria and/or hypertension at the first diagnosis, in 34 cases (10.7%); persistent microscopic hematuria with proteinuria in 32 cases (10.1%); and steroid resistance, steroid dependence, or frequent relapse forms of nephrotic syndrome in 32 cases (10.1%). Persistent microscopic hematuria was detected in 23 cases (7.2%), with positive family history, hypertension, increased urine RBC count, and parental anxiety.
3. Clinical diagnosis of patients before renal biopsy
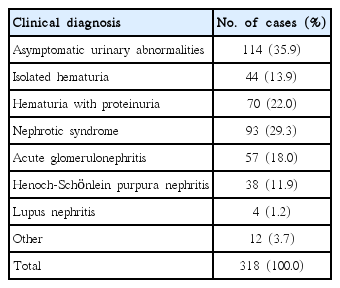
Prior to renal biopsy, the 318 cases were subdivided according to the following pre-biopsy clinical diagnoses (Table 1): 114 patients (35.9%) with asymptomatic urinary abnormalities, among whom 44 (13.9%) had isolated hematuria, 70 (22.0%) had hematuria with proteinuria; 93 patients (29.3%) with nephrotic syndrome; 57 patients (18.0%) with acute glomerulonephritis; 38 patients (11.9%) with Henoch-Schönlein purpura nephritis; 4 patients (1.2%) with lupus nephritis; and 12 patients (3.7%) with other conditions, such as acute renal failure, Alport syndrome, hemolytic uremic syndrome, and Korean hemorrhagic fever.
4. Histopathological diagnosis after renal biopsy
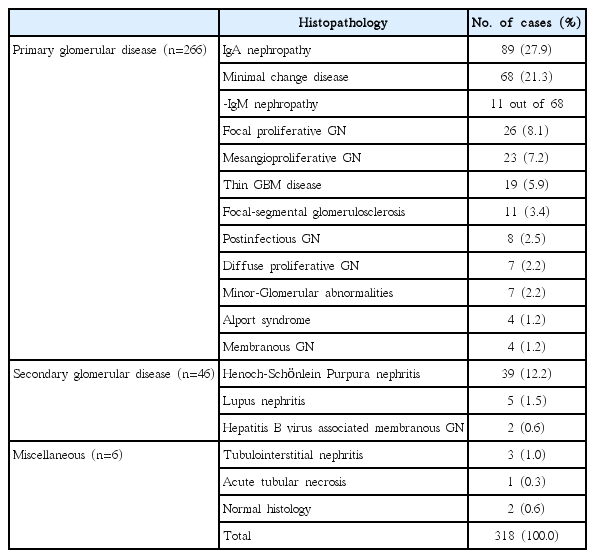
The distributions of histopathological diagnoses are summarized in Table 2. Glomerular disease was identified in 98.1% (312/318) of cases, with a diagnosis of primary glomerular disease in 85.2% (266/312) of these cases and of secondary glomerular disease in the remaining 14.7% (46/312). Common causes of primary glomerulonephritis (GN) were IgA nephropathy, minimal change disease (MCD), and focal proliferative GN, with Henoch- Schönlein purpura nephritis being the most common cause of secondary GN.
5. Histological classification of nephrotic syndrome
The distribution of histological diagnoses with nephrotic syndrome is summarized in Table 3. Among the 102 cases diagnosed with nephrotic syndrome, 97 cases were histologically classified as a primary nephrotic syndrome (95.1 %) and 5 cases (4.9%) as a secondary nephrotic syndrome. Among cases classified as a primary nephrotic syndrome, 68 cases (70.1%) presented with MCD, with IgM nephropathy accounting for 11 of these cases (16.2%). Focal segmental glomerulosclerosis (FSGS) was identified in 9 cases (9.3%), membranoproliferative GN (MPGN) in 7 cases (7.2 %), IgA nephropathy in 5 cases (5.2%), diffuse mesangial proliferative GN (DMPGN) in 4 cases (4.1%) and membranous glomerulopathy in 4 cases (4.1%).
6. Clinical status of nephrotic syndrome as glomerular morphology
Among patients diagnosed with nephrotic syndrome, microscopic hematuria was identified in 48 patients (47.1 %), as well as in 21 of the 68 patients (30.8%) with MCD, 6 of the 7 patients (85.7%) with MPGN and 6 of the 9 patients (66.7%) with FSGS (Table 3). Microscopic hematuria was identified in all patients with membranous glomerulopathy, postinfectious glomerulonephropathy, Henoch- Schönlein purpura nephritis, and nephropathy associated with hepatitis B viral infection. Hypertension was identified in 16 patients (15.7%), with decreased levels of serum complement in 12 patients (11.8%), and decreased serum albumin concentration of <2.5 gm/dL in 61 patients (59.8%), 51 of whom (83.6%) in the MCD group.
7. Clinical features according to histological grades in IgA nephropathy
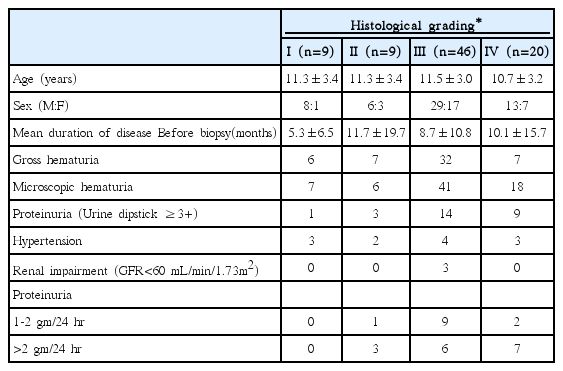
Based on histological findings by Haas M [6], IgA nephropathy was classified as grade I-V as in Table 4. In our study, 89 patients were diagnosed as having an IgA nephropathy following renal biopsy; a definite grade could not be established in 5 cases. The distribution of grades was as follows: 46 cases classified as grade III, 20 cases as grade IV, and 9 cases as grade I and II respectively. The male-to-female ratio of patients with IgA nephropathy was 2:1. Recurrent gross hematuria was identified in 52 cases (61.9%) with IgA nephropathy, with hypertension identified in 12 cases (14.2%), proteinuria >1.0 gm/24 hr in 28 cases (33.3%), and renal impairment in 3 cases (3.6%).
Discussion
In this study, we examined the anthropometric characteristics of patients who underwent renal biopsy along with their indications for renal biopsy and common histopathological findings. Our study revealed that the asymptomatic urinary abnormality is the most common clinical diagnosis and IgA nephropathy is the most common primary renal disease.
Renal biopsy in pediatric populations was introduced for diagnosing kidney disease in 1951 and has become a routine procedure in pediatric nephrology [7,8]. Over a 27-year period, 345 children underwent renal biopsy in our hospital and we identified an overall failure rate of renal biopsy of 1.2% (4/345), which is comparable to previously published rates [9,10]. Among our study sample, the male-to-female ratio was 1.8:1, a rate of male predominance that was comparable to previously reported sex-specific differences in prevalence [11]. Printza et al. [12] reported that 48.0% of pediatric patients who underwent renal biopsy were 11-14 years old, with 31.0 % between the ages of 6-10 years. We identified a similar age distribution, with 131 patients (41.2%) between the ages of 11-15 years and 129 (40.5%) between the ages of 6-10 years.
Previous studies have identified idiopathic nephrotic syndrome to the most common indication for renal biopsy [1316]. In contrast, 53.1% of patients in our study populations underwent renal biopsy to evaluate gross hematuria and/or microscopic hematuria. Since 1998 in South Korea, more than 4 million students have participated in annual large-scale urine screening programs; additionally, many school-children with asymptomatic hematuria and/or proteinuria were detected and their diagnoses confirmed through renal biopsy [17]. In current study, asymptomatic urinary abnormalities were clinically diagnosed before renal biopsy in 114 patients (35.9%), with 89 patients (27.9%) diagnosed as having IgA nephropathy after renal biopsy.
Nephrotic syndrome can be associated with various types of glomerulonephritis and, therefore, an accurate histopathological identification of the glomerular lesion is essential to establishing a correct diagnosis and providing the appropriate treatment. Kim et al. [18] reported that among 52 pediatric patients with nephrotic syndrome, 90.4% were diagnosed with primary nephrotic syndrome and 9.6% with secondary nephrotic syndrome. In our study, primary nephrotic syndrome was identified in 95.1% of cases, with secondary nephrotic syndrome identified in 4.9% of cases. According to Choi et al. [11], minimal change disease (MCD) was identified in 72.7% of all pediatric patients. A similar prevalence of MCD (70.1%) was found in our study. In addition, Keeping in mind the controversy regarding the nature of these IgM deposits [1921], MCD with mesangial IgM deposit was identified in 16.2% in our study. Microscopic hematuria is present at diagnosis in 20% to 30% of children with MCD but rarely persists; macroscopic hematuria occurs in less than 1% of children with MCD [22]. According to White et al. [23], microscopic hematuria was present in 13.0 % of cases with MCD. In our study, we identified hematuria in 30.8% of cases with MCD. Serum albumin levels usually fall below 2.0 g/dL and may be less than 1.0 g/dL in steroid sensitive nephrotic syndrome [24]. Kim et al. [18] reported that the prevalence of low serum albumin levels <2.5 g/dL was 78.4% in children with nephrotic syndrome. In our study, we found that 59.8% of children with nephrotic syndrome and 75.0% of children with MCD showed low serum albumin <2.5 g/dL. Elevated systolic and diastolic blood pressure are initially present in 5-20% of children with MCD, but hypertension usually does not persist [22]. However, the frequency of hypertension occurring in other types of nephrotic syndrome is as high as 80% [22]. In this study, only 15.7 % of all cases of nephrotic syndrome presented hypertension at diagnosis.
IgA nephropathy is the most prevalent form of glomerulonephritis worldwide. However, according to a large biopsy series, the prevalence of IgA nephropathy is influenced by significant race- and ethnicity-specific variation [6]. In their single center study, Choi et al. [11] reported a prevalence rate of IgA nephropathy of 18.2% in children, a prevalence rate similar to that reported in Japan [25]. However, the prevalence rate of IgA nephropathy in current study was 27.9%, relatively high compared to other studies. The higher prevalence of IgA nephropathy in our study reflects that many school children with asymptomatic urinary abnormalities have been detected through annual mass urinary screening, which started in 1998 in South Korea [17]. This screening program allows us to employ an active approach for evaluating children with persistent hematuria. Actually, the numbers of renal biopsy in our hospital increased annually compared to before 2000.
A majority of children with IgA nephropathy present with gross hematuria. In other patients, microscopic hematuria and/ or proteinuria are the only signs at presentation [26]. Asymptomatic gross hematuria was identified in 61.9% of children with IgA nephropathy in our study and the prevalence of gross hematuria is similar to other studies [27,28]. Although IgA nephropathy does not lead to significant renal damage, progressive renal damage develops in 20-30% of patients about 20 years after disease onset. Clinical parameters indicative of a poor prognosis are massive proteinuria (>1 gm/24 hr), renal insufficiency, and hypertension [29]. The prevalence of massive proteinuria (33.3%) and hypertension (14.2%) in present studied population are similar to that seen in other studies [27,28]. In addition, 23 of 27 patients (85.7%) with massive proteinuria and 7 of 12 patients (58.3%) with hypertension were diagnosed with a grade III or IV of IgA nephropathy. Because only less than 30% patients of IgA nephropathy have been follow-up more than 5 years, we could not identify a significant relationship between the above mentioned 3 clinical parameters and prognosis in children with IgA nephropathy.
In conclusion, the most common clinical diagnosis was asymptomatic urinary abnormalities, followed by nephrotic syndrome and acute glomerulonephritis. Primary glomerular disease is the most common histological finding, with IgA nephropathy being the most common histopathological lesion, followed by MCD. Our study demonstrates the importance of percutaneous renal biopsy in the diagnosis and treatment of pediatric renal diseases.
Notes
Conflict of interest
No potential conflict of interest relevant to this article was reported.