Introduction
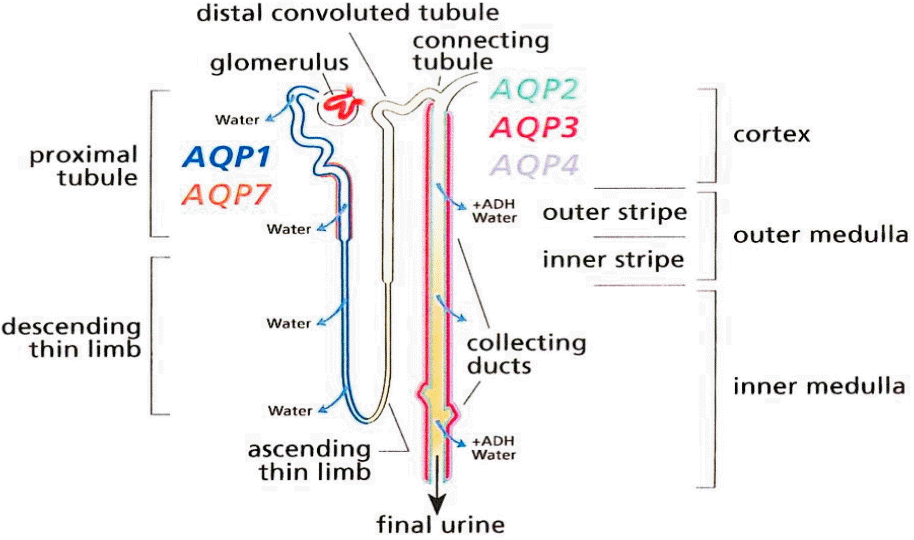
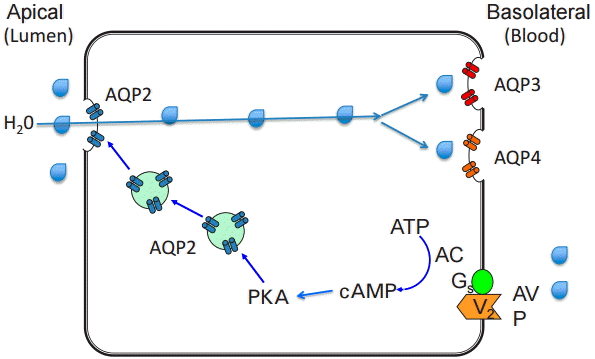
Water is an essential component for life for all plants and animals; however, cell membranes are composed of lipid bilayers that have low permeability to water. Therefore, simple diffusion by osmotic pressure is not sufficient for water to enter cells, and specialized transporters (aquaporins (AQP) that allow cells to absorb large amounts of water are needed to control the level of both extracellular and intercellular fluid (280-295 mOsmol/kgH2O in humans). AQP was first discovered by Peter Agre in 1992 [1]. The AQP protein is 30 kDa in weight, and is expressed in plants, bacteria, fungi, and mammals [2]. Thirteen types of AQP (AQP 0-12) have been identified in mammals. The majority of water absorption is via AQP1, localized in the proximal tubule and AQP2, expressed in the principal cells of the collecting tubule in the kidney (Fig. 1) [2-4]. Water reabsorption in the proximal tubule is coupled to solute reabsorption driven by the osmotic gradient [5]. Although 67% of the filtered water is reabsorbed in the proximal tubule (since AQP1 is not regulated by anti-diuretic hormone), the amount of water absorption in this segment does not control the final urine volume [2]. In contrast, AQP2 is localized in the principal cells that are also arginine vasopressin receptor target cells in the collecting tubule. AQP2 can be regulated by these hormones and controls the final urine volume [2]. The functional regulation of AQP2 thus becomes more important than AQP1. Indeed, mutations of the AQP2 caused several common diseases, including diabetes insipidus and noctturnal enuresis [2]. AQP2 surface expression and function is activated by arginine vasopressin (AVP). After AVP binds to the arginine vasopressin receptor 2 (V2R) in the cell basolateral membrane, intracellular cAMP is increased, which ultimately leads to the trafficking of AQP2 to the apical plasma membrane of collecting ducts (Fig. 2). Increased intracellular water absorbed by AQP2 is transported to the blood by AQP3 and AQP4 located in the basolateral membrane. The two main factors that regulate AQP2 are environmental osmolality and AVP [5]. Although the cortico-medullary osmolality gradient has a greater influence over AQP2 than AVP, the regulatory mechanisms controlling water balance through AQP2 are complex and are still not well understood. Interestingly, both aging has a significant effect on the expression of AQPs, and gender affect diurnal variation of plasma vasopressin and effect of desmopressin [6-8]. V2R expression and its ability to bind to AVP decreased by up to 30% in an aging rat model (F344BN rats) [6,9]. However, while AQP1 and 4 expressions were unchanged by aging, the expressions of AQP2 and 3 were decreased 80% and 50%, respectively, in 30-month-old rats compared to 10-month-old rats [9]. The circadian rhythm, which is controlled by the suprachiasmatic nucleus, is also known to decrease in the elderly [10]. Plasma levels of AVP are also higher in men compared to women, and the effects of vasopressin on the kidneys are greater in men [11].
The aim of this review is to evaluate the effect of different diets on AQP regulation and nighttime urine output. Information obtained from the available literature reports suggests that certain diets could be recommended to children with nocturnal enuresis, and may include avoiding water 2-3 hours before sleeping, a reduction in salt during dinner, and avoidance of high protein diets. This review also summarized the effects of hypercalciuria on AQP expression.
Effect of Water Loading and Deprivation on Urine Volume and AQP2
After a significant amount of water is loaded into the kidneys, plasma and urine osmolality is decreased and AQP2 expression is down-regulated to reduce renal water absorption [12]. In a previous study examining five healthy volunteers, urine osmolality decreased from 895┬▒38.5 to 86.3┬▒8.0 mOsm/kgH2O two hours after acute water loading (20 mL/Kg), urine volume increased from 0.68┬▒0.15 to 8.94┬▒3.14 mL/min, and urinary AQP2 was dramatically down-regulated from 266┬▒28 to 48┬▒17 pmol/mL [12]. In contrast, overnight dehydration decreased urine volume (1.0┬▒0.2 to 0.6┬▒0.1 ml/min), increased urine osmolality (888┬▒18 to 1004┬▒17 mOsmol/kg), and doubled urine AQP2 (140┬▒45 to 285┬▒63 fmol AQP2/╬╝moL creatinine) [13]. The results of these studies suggest that preventing water intake two hours before sleep should reduce night urine output.
AQP and a High Salt Diet
Many different studies have investigated the relationship between high and low sodium diets and the expression of AQP2 (Table 1) [14-17]. However, since the detection of renal AQP levels is difficult in humans, the studies have typically used urine AQP2 measures. Three percent of renal AQP2 is excreted daily into the urine, and urinary excretion of AQP2 per day was the same in men and women (mean age: 48.4┬▒15.8, 40.7┬▒16.6 years, average excretion: 380 pmol/d) [18]. This study suggested that because urine osmolality is positively correlated with urine AQP2 levels, interpreting AQP2 levels through spot urine samples should be carefully conducted [18]. Baumagarten et al. also suggested that because there is no simple relationship between urine osmolality and urine AQP2, random or singular measurement of urine AQP2 is not useful for evaluation of renal vasopressin activity [13]. Several previous studies have used a hypertonic saline infusion to mimic high sodium intake, and water deprivation and loading have also been used. Interestingly, even though urinary AQP2 excretion was increased by both water deprivation and hypertonic saline infusion, there were differences in the time it took for these changes to occur. The hypertonic saline infusion-induced change occurred more rapidly, within minutes, while the effects of dehydration took hours [19]. In a recent human study on the effect of high and low sodium intake on AQP2, urine AQP2 concentration increased from a baseline of 113 to 144 ng/mmol after a high sodium diet (300 mmol sodium/day; 17.5 g salt/day for 4 days) and hypertonic saline infusion (3%, 7 ml/kg) [16]. Urine volume (7.6-8.4 ml/min) was decreased by both a high salt diet (2.7-3.4 ml/min) and a low salt diet (1.5-2.1 ml/sec). Interestingly, urine AQP2-creatinine was slightly increased from 79 to 84-102 ng/mmol by a low sodium diet (30 mmol sodium/day; 1.8 g salt/day for 4 days) [16]. While blood pressure was slightly increased by a high sodium diet, angiotensin II and aldosterone, which are the important regulators of reabsorption of sodium in the kidney, decreased from 13.6 to 9.3 pmol/l and 225 to 148 pmol/l, respectively [16]. These results suggest that if plasma osmolality is increased by a high salt diet, renal sodium excretion is increased by the down-regulation of angiotensin II and aldosterone, which subsequently increases water reabsorption to reduce blood osmolality. However, another study reported different results and found that a 2.59% NaCl diet increased urine volume (40┬▒ to 81┬▒7 ml/day/kg/BW) and AQP2 despite similar AVP levels (0.6┬▒0.3 to 0.4┬▒0.1 pmol/l) [20]. In addition, Roxas et al. fed either a normosodic diet (0.4% NaCl) or a high-sodium diet (8%) for 10 days to male Sprague Dawley rats and Dahl SS/Jr rats [17]. While AQP1 and AQP2, measured by RT-PCR, were significantly decreased (5 fold lower) in Sprague Dawley rats in the high-sodium diet group, AQP2 expression was three-fold higher in Dahl SS/Jr rats treated with a high salt diet. They suggested that the reduction in AQP-2 expression may be a compensatory mechanism for reducing salt and water reabsorption after a high salt diet in Sprague Dawley rats. The increase in AQP2 in Dahl SS/Jr rats may be a mechanism to increase salt and water reabsorption that has arisen through AQP2 mutation or polymorphism of AQP2 in this rat model, and could be linked to hypertension [17].
In a similar study, Penna et al. reported that a high-sodium diet (8% NaCl) increased angiotensin II, TGF-╬▓1, and ╬▒-SMA, and decreased AQP-1, AQP-2, and eNOX expression in male Sprague Dawley rats [15]. In addition, losartan (40 mg/kg/day), an AT1 receptor blocker, prevented this effect. Unfortunately, the relationship between urine AQP2 and serum AVP during a high salt diet or hypertonic saline infusion is also unclear. The changes in urine AQP2 rate and AVP in healthy humans were positively correlated during water deprivation and hypertonic saline infusion [19]. In contrast, Saito et al. reported that a 5% saline infusion in five young controls resulted in an elevation of plasma AVP (1.0 ┬▒0.2 to 4.8┬▒0.7 pg/mL) due to an increase in plasma osmolality, and a decrease in urine volume and urinary AQP2 [21]. Elliot et al. reported different results from previous studies, and found that urine volume increased from 76┬▒15 to 156┬▒29 mL/h in 90 min after a 3% NaCl infusion in healthy volunteers [22]. The significant discrepancy between these studies raises several questions namely, why does urinary AQP2 decrease while plasma AVP increases, and why does urine volume decrease while urinary AQP2 decreases? Although the exact mechanisms are still unclear, there are several possible explanations. The studies mentioned here have been conducted in multiple different species, including various kinds of rats and humans. The basic mechanism for absorption and excretion of sodium and water is relatively similar between these species, but there are some important differences. Furthermore, the response of AQP2 after water loading or deprivation differs by time. For example, urinary AQP2 levels are lowest two hours after water loading, and then increase for up to four hours, although absolute levels still remain lower than baseline [12]. Furthermore, no studies have been conducted in children. In addition, and most importantly, the amount of water given can significantly affect the results because both plasma and urine water/sodium concentration (osmolality) are significantly influenced by the regulation of AVP and AQPs. The plasma concentration of AVP in rats may have decreased because they could freely access water. AQP concentration could have decreased due to an excess of water, and because plasma osmolality was increased by the hypertonic saline infusion and/or high salt diet in the human study. AQP2 levels and renal absorption may be increased to reduce plasma concentration. Therefore, different conditions after a high salt diet can variably influence urine volume and AQP. As previously mentioned, we have to consider the limitations of urine AQP2 sampling in the evaluation of renal AQP2. Finally, many other molecules, including angiotensin II/aldosterone, ENaC, and AQP1, also play important roles in the control of sodium, water and blood pressure.
Effect of Fasting
The renal response to fasting differs from that of water deprivation. Although the effects of acute fasting have not been reported, fasting for 24 hours reduced urine AQP2 (14%) and increased urine output [23]. Because fasting increases prostaglandin synthesis, and because the inhibition of prostaglandin can increase the antidiuretic activity of vasopressin, many studies have been conducted using NSAIDs. However, study results have shown inconsistent effects of prostacyclin and NSAIDs on AQP2 [24,25].
Effect of Low Potassium Diet
The kidney and muscle have a role in compensation for acute effects of low potassium diet on urine volume and AQP2 levels. Amlal et al. reported that urine osmolality decreased in as little as 12 hours after beginning a potassium free diet, and that urine volume increased after 24 hours [26]. Interestingly, whereas an early urinary concentrating defect was induced from the down-regulation of cortical AQP2, later onset of the same defect resulted from decreased medullary AQP2 [26]. A long-term low potassium diet has been reported to lower urine osmolality by about 50%, to double urine excretion volume, and decrease AQP-2 levels by 50% [26,27].
Effect of High Protein Diet
High protein diets are included in this review because protein intake is a factor in osmotic diuresis. In addition to electrolytes, such as sodium and potassium, urea from a high protein diet and glucose are the main solutes responsible for osmotic diuresis [28,29]. Total solute loss is higher than the loss of water in osmotic diuresis, and serum osmolality decreases [29]. Previous studies have shown the effects of high and low-protein diets on the expression of AQP2 [30-32]. Urine concentration decreased after a low-protein diet in both humans and rats. A decrease in AQP2 protein expression was also accompanied by reduced urine concentrating ability [30,31]. In contrast, a high-protein diet led to increased absorption of water and increased urine AQP2 (92.5 to 164.0 ng/╬╝mol creatinine), but there was no change in urine volume between a normal (2,423 mL/24 h) and protein enriched diet (2,376 mL/24 h) [32]. Urine sodium excretion increased from 52 on a normal diet to 75 mmol/24 h on a high protein diet [32]. Urine prostaglandin levels were also reduced by a high protein diet (690 to 543 pg/min); however, while AVP and ANGII up-regulate AQP2, prostaglandin downregulates AQP2 expression [32].
Effect of Hypercalciuria
Hypercalciuria is one of the causes of voiding dysfunction in children, including nocturnal enuresis and daytime frequency syndrome. The effect of hypercalciuria on AQP2 was investigated in 80 children with NE and 9 controls, 24 hour urine (day and night) was collected in control and NE groups [33]. The NE subjects were divided into three groups (G1: low vasopressin levels and nighttime diuresis, G2: low vasopressin levels and balanced diuresis, G3: normal vasopressin and daytime diuresis). The results showed that the day/night AQP2 ratio was approximately three-fold higher in G1 subjects who displayed hypercalciuria (1.67┬▒0.41) compared to healthy children (0.59┬▒0.11), and the ratio was approximately two-fold higher in normocalciuric patients with NE (1.27┬▒0.24) [33]. A study was recently published on how NE can be improved through a low calcium diet using vasopressin in children with NE and hypercalciuria [34]. The same research group recently reported that there is a relationship between urinary calcium excretion and AQP2 [35]. Hypercalciuria was induced by bone demineralization for 7 days of adaptation and 24 days of bed rest in 10 healthy men [35]. Urinary calcium excretion increased in the first 7 days, and then gradually decreased over 35 days. AQP2 excretion increased in the first 7 days, decreased through day 14, and then increased again until day 35 [35]. One possible explanation for this result is that the increased urinary calcium excretion may temporarily down-regulate AQP2, and since plasma volume is reduced by increased water excretion, AQP2 levels recover after calcium excretion is normalized [35].
Conclusion
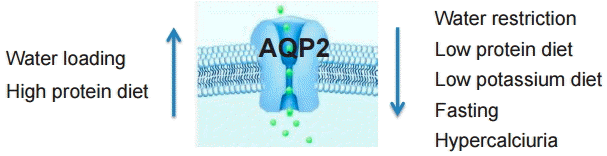
The expression of renal AQP2 is mainly regulated by the absorption and excretion of sodium and water. Plasma concentration of other electrolytes, such as potassium and calcium, and nutrients, including protein, ingested through the diet can also regulate AQP2 concentration. Other regulatory factors, including AVP, PGE2, ENaC, and renin/ANGII/aldosterone, are also involved in this regulatory process. It is relatively clear that AQP2 is upregulated by water loading and high protein diet intake and down regulated by water restriction, low protein diet, low K diet and fasting. AQP2 is also down regulated by hypercalciuria (Fig. 3). However, the regulation of AQP2 by Na intake is contradictory as shown in table 1. The contradictory results may be caused by many factors, such as different experimental conditions, and compensatory mechanisms that regulate AQP2. Urine sampling of AQP2 in spot urine samples has also limited ability to reflect renal AQP2 concentration, and results differ significantly depending on the time to evaluation and duration of specific diets. Because drinking a significant amount of water can induce maximum diuresis after 2 hours, reduced water intake could greatly help to reduce nighttime diuresis. The effect of a high salt diet on AQP2 expression was shown to be inconsistent in previous studies, and therefore requires further investigation. If amount of protein intake is not significant such as parenteral nutrition and/or combined with other solute and/or water diuresis, because increased AQP2 after high protein diet can increase water absorption, it may not be an important cause for diuresis in children with NE. Hypercalciuria can reduce AQP2 and increase urine volume, so reducing intake of calcium rich foods may also help reduce nighttime diuresis.



 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Download Citation
Download Citation Print
Print