| Child Kidney Dis > Volume 20(2); 2016 > Article |
|
Abstract
Purpose
Disruption of normal renal development can lead to congenital anomalies of the kidney and urinary tract, including renal hypodysplasia. We aimed to clarify whether small kidney size affects clinical manifestations in children with urinary tract infection (UTI).
Methods
One hundred fifty-four patients who had their first symptomatic UTI between January 2014 and June 2015 were enrolled in this study. Differences in kidney size were estimated based on percent uptake of 99mTc-dimercaptosuccinic acid (DMSA) in scintigraphy. The patients who showed more than 10% difference in kidney size on DMSA scintigraphy with none or minimal cortical defects were included in group A. (group A, n=17). Laboratory, clinical, and imaging results were compared with those of the other patients (group B, n=137).
Results
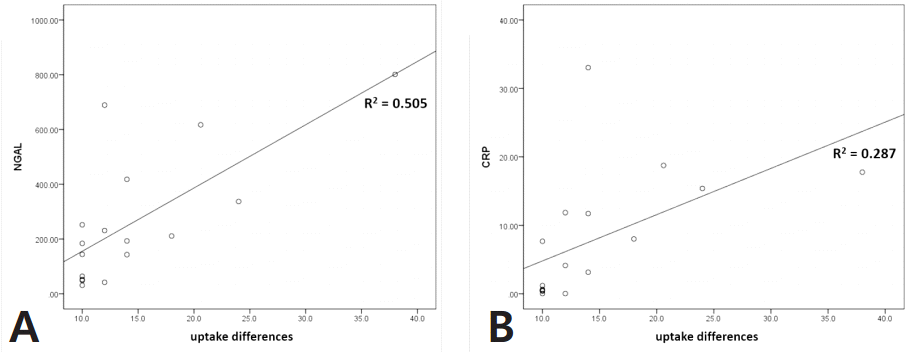
Group A had a relatively higher incidence of vesicoureteral reflux than group B (44% vs 20%, P <0.05). The levels of plasma neutrophil gelatinase-associated lipocalin (NGAL) and serum C-reactive protein were significantly higher in group A (193 [64-337] vs 91 [59-211] ng/mL and 4.1 [0.5-11.9] vs 2.1 [0.7-5.3] ng/ mL, respectively; all P <0.05). Linear regression analysis revealed that plasma NGAL level strongly correlated with the difference in renal uptake in DMSA scintigraphy in group A (R2=0.505).
Congenital abnormalities of the kidney and the urinary tract (CAKUT) are among the most frequent antenatal malformations and occur in 1/500 live births [1]. Disruption of renal development can lead to CAKUT, including renal hypoplasia, which is characterized by congenitally small kidneys with dysplastic features and a reduced number of nephrons [2,3]. Renal hypoplasia can be diagnosed as a small-sized kidney of 2 standard deviations below normal [4]. In several studies, mild renal hypoplasia at the lower normal range is associated with various nephrouropathies, including more frequent urinary tract infection (UTI) [2,4].
In diagnosing renal hypoplasia in children, the volume of the kidneys should be measured. The most widely used method for estimating kidney volume is kidney length measurement using ultrasonography [5,6]. However, intra-observer and inter-observer variation may occur when ultrasonography is used on children [7], and calculating volume based on length may present a limitation in assessing the kidney size of a pediatric patient [5].
99mTc dimercaptosuccinic acid (DMSA) renal scintigraphy is considered the standard method for assessing the extent and progression of renal parenchymal damage in UTI patients [8-10]. Furthermore, estimation of kidney function, along with kidney size, is possible based on the level of DMSA scintigraphy uptake, with the normal range for the relative uptake of each kidney being between 45% and 55% [11-13].
In the present study, kidney size in children with UTI was estimated based on the level of uptake on DMSA scintigraphy. Using these results, the present study examined clinical features of UTI and issues associated with small kidneys.
The present study was retrospective in design including patients admitted to Korea University Hospital between January 2014 and June 2015 for their first symptomatic UTI. Among all patients who were admitted for UTI during this period, only those who underwent both DMSA scintigraphy and laboratory tests were included in the study. A febrile UTI was defined as the presence of fever (body temperature Ōēź38Ōäā), pyuria (Ōēź5 WBCs/high-power field), and a positive urine culture (pure bacterial growth of >105 colony forming units/mL). Patients with a history of UTI or any other infectious diseases were excluded, along with those with any congenital anomaly other than VUR, renal hypoplasia, and small kidney. We divided patients into two groups, Group A and B. Group A included patients who showed the following: 1) more than 10% uptake difference of the right and left kidney in DMSA renal scintigraphy and, 2) the difference could not be from a pyelonephritic uptake defect. Patients that did not meet these criteria were defined as Group B.
According to our department protocol, the measurement of plasma neutrophil gelatinase-associated lipocalin (NGAL), serum procalcitonin (PCT), serum C-reactive protein (CRP), and white blood cell (WBC) counts were performed at the time of admission. Renal ultrasonography and DMSA scintigraphy were performed on all enrolled patients within the first 3 days after admission. A voiding cystourethrogram was performed before patients were discharged only if DMSA scintigraphy detected a cortical defect or vesicoureteral reflux (VUR) was clinically suspected.
The injected dose of DMSA scintigraphy was scaled according to body surface area [14].
All patients were placed in the supine position, and examined using a high resolution, low energy collimator. After tracer injection, four images (anterior, posterior, and left and right posterior oblique views) were acquired. An experienced observer performed the visual analysis of the images following the procedure guidelines for renal scintigraphy [12,15]. Hydronephrosis was diagnosed according to the Society for Fetal Urology classification [16]. The laboratory, clinical, and imaging results were compared between the two groups.
Statistical comparisons were performed with the SPSS program for Windows (version 18.0, SPSS, Chicago, IL). Continuous variables between groups were compared using the StudentŌĆÖs t-test and categorical variables were compared using the chi-squared test. Data are presented as the median (interquartile range [IQR]) for continuous variables and proportions for categorical variables. Statistical significance was set at P<0.05. Scatter plots and linear regression analysis were used to determine the correlations between the uptake differences and biomarkers, and analysis was performed separately for all patients and Group A only.
Among 202 patients, 48 were excluded according to the study criteria; therefore, a total of 154 patients participated in the study. Among them, 17 (11%) were assigned to Group A. The age range of the two groups varied from 1 month to 13 years old, but there was no statistically significant difference in mean age between the two groups (Group A, 28 months [IQR: 5-43]; Group B, 20 months [3-19]). Moreover, the two groups did not show statistically significant differences in gender and fever duration. Among the biomarkers, levels of plasma NGAL (193 [range, 64-337] ng/mL vs. 91 [range, 59-211] ng/mL) and serum CRP (4.1 [range, 0.5-11.9] ng/mL vs. 2.1 [range, 0.7-5.3] ng/mL) and WBC counts (18,630 [range, 15,920-20,750]/mm3 vs. 14,100 [range, 9,500-17,540]) showed statistically significant differences between the two groups (all P<0.01). The percentages of patients diagnosed with acute pyelonephritis (APN) or hydronephrosis did not show a statistically significant difference between Group A and B (59% vs. 49% and 53% vs. 37%, respectively), whereas VUR was slightly higher in Group A (44% vs. 20%, P<0.05) (Table 1).
Scatter plot and linear regression were used to determine whether biomarker levels increase according to uptake difference. The results from linear regression analysis performed on all patients showed weak positive correlations with plasma NGAL and serum CRP only (R2=0.155 and 0.124) (Fig. 1). Analysis limited to Group A only showed similar results, but the correlation between plasma NGAL and uptake difference was more significant (R2=0.505) (Fig. 2).
Febrile UTI is common in pediatric patients. Approximately 7-8% of girls and 2% of boys have a UTI during the first 8 years of life [17,18]. UTIs can occur as simple bladder infections, or involve the kidneys, leading to renal scarring. Furthermore, kidney injury related to APN has been considered a cause of substantial long-term morbidity [19]. Retrospective studies have suggested that kidney injury related to UTI presents a clinically significant risk, with high subsequent rates of chronic kidney disease, hypertension, and preeclampsia [20,21].
Since the symptoms of UTI in children are nonspecific, diagnosis of APN in patients with UTI often requires examination with DMSA scintigraphy to detect renal parenchymal involvement [22,23]. The level of uptake obtained from DMSA scintigraphy enables kidney function and size to be quantified [24,25]. Currently, some biomarkers are considered more practical and accessible tools capable of estimating kidney injury [26].
NGAL is a 25-kDa protein originally purified from human neutrophils [27]. This protein, which is released from developing nephrons, participates in the conversion of metanephric tissue into glomeruli and proximal renal tubules [28]. NGAL is also expressed at low levels in normal organs and at increased levels in injured epithelia, including the kidney [29]. In recent studies, plasma NGAL has been identified as one of the most reliable biomarkers of kidney injury in pediatric UTI [30-32].
In the present study, Group A and B did not differ significantly with respect to hydronephrosis and APN. Although VUR was slightly more common in Group A, the difference was very small. Radiological diagnosis did not show differences between the two groups; however, plasma NGAL, a biomarker directly associated with kidney injury in UTI, was higher in Group A, showing strong significance.
It is believed that these results may be attributed to the limitation of APN assessment via DMSA scintigraphy. Even though it is considered the gold standard for diagnosis of APN, DMSA scintigraphy was limited in identifying renal cortical lesions [33,34]. In other words, APN with no cortical defect may be underestimated due to false negative results on DMSA scintigraphy. However, since plasma NGAL expression is increased in cases with injury to the renal tubular epithelium caused by bacterial infection [35,36], NGAL expression level also increases in APN without renal cortical defects. Therefore, higher plasma NGAL in Group A suggests that patients with small kidneys are more prone to kidney injury during UTI or APN.
Furthermore, a greater difference in uptake on DMSA scan was associated with increased biomarker levels. In particular, when Group A patients showed a difference Ōēź10 %, the association increased even more. This result could be interpreted as meaning that a smaller kidney sustains a more severe injury.
The present study had some limitations. First, the study was limited to only children at risk for UTI; therefore, generalizing the findings in the study to all children would be problematic. Second, kidney size measurements were only performed with DMSA scintigraphy. This can lead to uncertainty about measurement of kidney size based on cortical uptake, even if a patient selection process did take place. For measuring kidney size more accurately, testing with additional methods such as ultrasonography or computed sonography is needed. Third, this was a single-center study with a small number of registered patients. Therefore, more accurate and generalizable conclusions require multicenter studies with larger sample sizes.
References
1. Song R, Yosypiv IV. Genetics of congenital anomalies of the kidney and urinary tract. Pediatric Nephrology 2011;26:353-364.


2. Quirino IG, Diniz JSS, Bouzada MCF, Pereira AK, Lopes TJ, Paix├Żo GM, et al. Clinical course of 822 children with prenatally detected nephrouropathies. Clin J Amer Soc Nephrology 2012;7:444-451.

3. Andr├®s-Jensen L, J├Ėrgensen FS, Thorup J, Flachs J, Madsen JL, Maroun LL, et al. The outcome of antenatal ultrasound diagnosed anomalies of the kidney and urinary tract in a large Danish birth cohort. Arch Dis Childhood 2015;309784.

4. Cain JE, Di Giovanni V, Smeeton J, Rosenblum ND. Genetics of renal hypoplasia: insights into the mechanisms controlling nephron endowment. Pediatr Res 2010;68:91-8.


5. Dinkel E, Ertel M, Dittrich M, Peters H, Berres M, Schulte-Wissermann H. (1985) Kidney size in childhood sonographical growth charts for kidney length and volume. Pediatric Radiology 2010;15:38-43.

6. Han BK, Babcock DS. Sonographic measurements and appearance of normal kidneys in children. Amer J Roentgenology 1985;145:611.

7. Schlesinger A, Hernandez R, Zerin J, Marks T, Kelsch R. Interobserver and intraobserver variations in sonographic renal length measurements in children. Amer J Roentgenology 1991;156:1029-32.

8. Rushton HG. The evaluation of acute pyelonephritis and renal scarring with technetium 99m-dimercaptosuccinic acid renal scintigraphy: evolving concepts and future directions. Pediatric Nephrology 1997;11:108-20.


9. Lavocat MP, Granjon D, Allard D, Gay C, Freycon MT, Dubois F. Imaging of pyelonephritis. Pediatric Radiology 1997;27:159-65.


10. Benador D, Benador N, Slosman DO, Nussl├® D, Mermillod B, Girardin E. Cortical scintigraphy in the evaluation of renal parenchymal changes in children with pyelonephritis. J Pediatrics 1994;124:17-20.

11. Piepsz A, Blaufox M, Gordon I, Granerus G, Majd M, O'reilly P, et al. Consensus on renal cortical scintigraphy in children with urinary tract infection. Seminars in Nuclear Medicine. Elsevier 1999;160-74.
12. Piepsz A, Colarinha P, Gordon I, Hahn K, Olivier P, Roca I, et al. Guidelines for 99mTc-DMSA scintigraphy in children. European J Nuclear Med 2001;28:BP37-41.
13. Piepsz A, Tamminen-M├Čbius T, Reiners C, Heikkil├ż J, Kivisaari A, Nilsson N, et al. Five-year study of medical or surgical treatment in children with severe vesico-ureteral reflux dimercaptosuccinic acid findings. Euro J Pediatrics 1998;157:753-8.

14. Piepsz A, Hahn K, Roca I, Ciofetta G, Toth G, Gordon I, et al. A radiopharmaceuticals schedule for imaging in paediatrics. Euro J Nuclear Med 1990;17:127-9.

15. Mandell GA, Eggli DF, Gilday DL, Heyman S. Procedure guideline for renal cortical scintigraphy in children. J Nuclear Medicine 1997;38:1644.
16. Fernbach S, Maizels M, Conway J. Ultrasound grading of hydronephrosis: introduction to the system used by the Society for Fetal Urology. Pediatric Radiology 1993;23:478-80.


17. Hellstr├Čm A, Hanson E, Hansson S, Hj├żlm├źs K, Jodal U. Association between urinary symptoms at 7 years old and previous urinary tract infection. Arch Dis Childhood 1991;66:232-4.

18. M├źrild S, Jodal U. Incidence rate of firstŌĆÉtime symptomatic urinary tract infection in children under 6 years of age. Acta Paediatrica 1998;87:549-52.


19. Montini G, Tullus K, Hewitt I. Febrile urinary tract infections in children. New England J Med 2011;365:239-50.

20. Jacobson SH, Ekl├Čf O, Lins L-E, Wikstad I, Winberg J. Long-term prognosis of post-infectious renal scarring in relation to radiological findings in childhood a 27-year follow-up. Pediatric Nephrology 1992;6:19-24.


21. Zhang Y, Bailey R. A long term follow up of adults with reflux nephropathy. New Zealand Medical J 1995;108:142-4.
22. Baumer J, Jones R. Urinary tract infection in children, National Institute for Health and Clinical Excellence. Arch Dis Childhood-Education Practice Ed 2007;92:189-92.

23. Newman TB. The new American Academy of Pediatrics urinary tract infection guideline. Pediatrics 2011;128:572-5.


24. Estorch M, Torres G, Camacho V, Tembl A, Prat L, Mena E, et al. Individual renal function based on 99mTc dimercaptosuccinic acid uptake corrected for renal size. Nuclear Med Comm 2003;25:167-70.

25. Rossleigh M. A., Farnsworth R. H., Leighton D. M., Yong J. L.. Technetium-99m dimercaptosuccinic acid scintigraphy studies of renal cortical scarring and renal length. J Nuclear Med 1998;39:1280-5.
26. Yim HE, Yim H, Bae ES, Woo SU, Yoo KH. Predictive value of urinary and serum biomarkers in young children with febrile urinary tract infections. Pediatric Nephrology 2014;29:2181-9.


27. Kjeldsen L, Johnsen AH, Sengel├Ėv H, Borregaard N. Isolation and primary structure of NGAL, a novel protein associated with human neutrophil gelatinase. J Biol Chem 1993;268:10425-32.

28. Mori K, Nakao K. Neutrophil gelatinase-associated lipocalin as the real-time indicator of active kidney damage. Kidney International 2007;71:967-70.


29. Supavekin S, Zhang W, Kucherlapati R, Kaskel FJ, Moore LC, Devarajan P. Differential gene expression following early renal ischemia/reperfusion. Kidney International 2003;63:1714-24.


30. Bolignano D, Donato V, Coppolino G, Campo S, Buemi A, Lacquaniti A, et al. Neutrophil gelatinaseŌĆōassociated lipocalin (NGAL) as a marker of kidney damage. Amer J Kidney Dis 2008;52:595-605.

31. Seo WH, Nam SW, Lee EH, Je B-K, Yim HE, Choi BM. A rapid plasma neutrophil gelatinase-associated lipocalin assay for diagnosis of acute pyelonephritis in infants with acute febrile urinary tract infections: a preliminary study. European J Pediatrics 2014;173:229232.

32. Sim JH, Yim HE, Choi BM, Lee JH, Yoo KH. Plasma neutrophil gelatinase-associated lipocalin predicts acute pyelonephritis in children with urinary tract infections. Pediatric Res 2015;78:48-55.

33. Lee J, Kwon DG, Park SJ, Pai K-S. Discordant findings on dimercaptosuccinic acid scintigraphy in children with multi-detector row computed tomography-proven acute pyelonephritis. Korean J Pediatrics 2011;54:212-8.

34. Lee SW, Baek SY, Lee SJ. CT of acute pyelonephritis in children: comparison with Tc-99m DMSA scintigraphy. J Korean Radiological Soc 1998;38:933-9.

Fig.┬Ā1.
Scatter plots and linear regression analysis of total patients regarding uptake differences with plasma NGAL (A, R2 =0.155) and serum CRP (B, R2 =0.124).

Fig.┬Ā2.
Scatter plots and linear regression analysis of Group A regarding uptake differences with plasma NGAL (A, R2 =0.505) and serum CRP (B, R2 =0.287).
Table┬Ā1.
Patient Characteristics
- TOOLS
-
 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link XML Download
XML Download Full text via DOI
Full text via DOI-
 Download Citation
Download Citation
- Download Citation
-
- Close
 Print
Print-
Share :
-
METRICS

-
- 1 Crossref
- 0 Scopus
- 12,493 View
- 96 Download
- Related articles
-
Clinical Significance of Electrolyte Imbalance in Pediatric Urinary Tract Infection2011 April;15(1)




