Experience with Pediatric Kidney Transplantation, 1985-2016: A Single Regional Center Study
Article information
Abstract
Purpose
Kidney transplantation (KT) is an ideal treatment for pediatric patients with end-stage renal disease (ESRD). We report the clinical outcomes of pediatric ESRD patients who underwent KT in a single regional center.
Methods
We retrospectively investigated the medical records of 60 pediatric patients who were diagnosed with ESRD and underwent KT in our hospital between January 1985 and June 2016.
Results
A total of 60 children and adolescents (40 male, 20 female; mean age, 13.86±4.26 years) were included in this study. Six patients (10.0%) underwent KT immediately after receiving the diagnosis of ESRD, while the others underwent KT after dialysis treatment (mean period of dialysis, 368.7±4,41.8 days). The mean donor age (50 living-related [83.3%], 10 deceased [16.7%]) was 40.0±12.85 years and the male:female ratio was 1.07:1. The most common cause of ESRD was chronic glomerulonephritis. The overall survival rates at 1, 3, and 5 years after KT were 98%, 98%, and 96%, respectively, while the graft survival rates at 1, 3, and 5 years were 93%, 86%, and 68%, respectively. Children who underwent KT before 10 years of age had better monthly growth rates than those who underwent KT later than 10 years of age.
Conclusions
KT is performed less frequently in children than in adults, but causes of ESRD vary and clinical outcomes after KT greatly affect the growth and development of pediatric patients. Therefore, further analysis and monitoring of clinical progression after KT in pediatric ESRD patients are necessary.
Introduction
Over the past three decades, despite many advances in renal replacement therapies, kidney transplantation (KT) remains one of the most ideal treatments for pediatric patients with end-stage renal disease (ESRD) [1-3]. KT provides freedom from dialysis and is cost-effective for providing great opportunities for normal growth and development in pediatric patients with ESRD [3-6]. In addition, the risk of death is more than four times higher with dialysis than with KT [1]. The first KT in Korean children was performed in 1973 [7,8]. Poorer graft survival in KT during childhood was reported due to the nature of pediatric KT in terms of inconsistent adherence to medication regimens, worse side effects of medications, higher rates of graft rejection due to recurrent disease, and the more intense immunoreactivity of children [9]. However, improvements in immunosuppressive management, surgical techniques, and long-term supportive care have had a favorable impact on pediatric KT results [8]. The 5-year survival rate of pediatric KT has recently been similar to that of adults, but there is an important difference between pediatric and adult KT in that the result of the former greatly affects pediatric growth and development [8,9]. However, the clinical data of pediatric KT versus adult KT in Korea has not been systematically established yet. Therefore, here we report the clinical outcomes of pediatric KT performed in a single regional center.
Material and methods
We retrospectively reviewed the medical records of 60 pediatric patients <20 years of age who were diagnosed with ESRD and underwent KT at Kyungpook National University Hospital between January 1985 and June 2016. The data included information about the recipients and donors: recipient age, sex, underlying renal disease, dialysis type, dialysis duration, and immunosuppressants used for induction therapy; donor age and sex; and the relationship of the recipient and donor. The survival analysis of graft failure (graft survival analysis) and mortality (patient survival analysis) were performed using the Kaplan-Meier method, while the statistically significant differences between the two groups were assessed by the log-rank test (using R software version 3.3.1).
Results
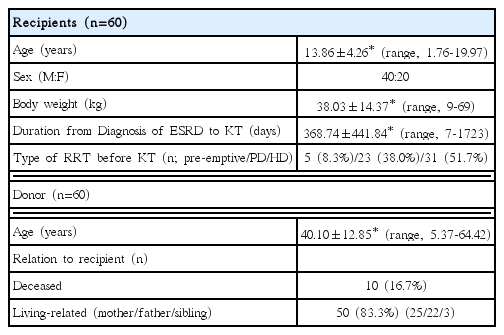
Between January 1985 and June 2016, 60 children and adolescents <20 years of age were diagnosed with ESRD and underwent KT in our hospital. Of them, 40 were male (66.7%) and 20 were female (33.3%) with a mean age of 13.86±4.26 years (range, 1.76-19.97 years). The youngest patient at the time of KT was a 1.76-year-old girl whose body weight was 9 kg. Six patients (10.0%) underwent KT immediately after receiving the diagnosis of ESRD, while the others underwent KT after dialysis (mean dialysis duration, 368.7±441.8 days; 23 for peritoneal dialysis and 31 for hemodialysis) (Table 1).
The causes of ESRD were as follows: 20 with chronic glomerulonephritis (33.3%; seven focal segmental glomerulosclerosis, four with immunoglobulin A nephropathy, four with membranoproliferative glomerulonephritis, three with Henoch-Schönlein nephritis, two with interstitial nephritis), seven with reflux nephropathy (11.7%), four with hereditary kidney disease (6.7%), three with polycystic kidney disease (5.0%), one with kidney dysplasia (1.7%), four with others (6.7%), and 21 unknown (35.0%) (Table 2).
The mean donor age was 40.10±12.85 years (range, 5.37- 64.42 years), while the male: female ratio was 1.07:1. Ten donors (16.7%) were deceased and 50 donors (83.3%) were living-related (including 25 mothers, 22 fathers, and three siblings) (Table 1).
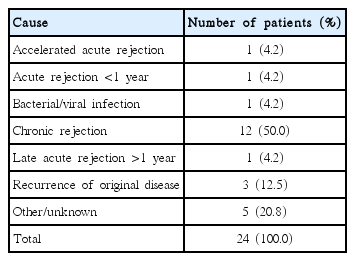
The graft survival rates of 60 patients at 1, 3, and 5 years after KT were 93%, 86%, and 68%, respectively, while the patient survival rates of them at 1, 3, and 5 years after KT were 98%, 98%, and 96%, respectively (Table 3). The graft survival rates of deceased donor recipients at 1, 3 and 5 years after KT were 100%, 89%, and 59% and those of living donor recipients were 91%, 85%, and 70%. The most common cause of graft failure was chronic rejection (n=12; 50%), while the second most common cause was recurrence of the original renal disease (n=3; 12.5%) (Table 4). A total of five patients died, and the known causes of death were bacterial infection and hemorrhage. 26 patients (44.1%) had acute rejection after KT and one of them had graft failure.
Since 2000, tacrolimus has been used as an initial major immunosuppressant therapy instead of cyclosporine and basiliximab (SimulectⓇ) has been used as the induction therapy for grafts from deceased donors in our center. However, there was no significant difference in patient and graft survival rates between patients who received KT before and patients who received KT after 2000. Similarly, there was no significant difference between the deceased donor recipients group and living donor recipients group in patient or graft survival analysis (Table 3). Estimated graft survival probabilities were 91%, 85%, and 73% at 1, 3, and 5 years, respectively, for cyclosporine therapy. Corresponding estimates for tacrolimus therapy were 95%, 95%, and 64%. No significant differences were seen between the two distinctive therapy groups in graft or patient survival analysis.
There reported 2% of post-transplantation lymphoproliferative disorder (PTLD) (n=1) after KT. The most common post-transplant infection was cytomegalovirus (CMV) infection. There were 4 CMV infected patients. 3 of them (5.2%) had symptoms and culture proven viremia, 1 patient (1.7%) had viremia only. No patient had Epstein-Barr virus (EBV) infection and 2 patients had BK virus infection.
The height percentage change per month differed significantly according to KT timing. The children who underwent KT before 10 years of age showed a 0.15±0.2% increase in growth rate, whereas the children who underwent KT after 10 years of age showed a -0.05±0.17% growth rate change (P=0.02) (Fig. 1).
Discussion
ESRD occurs in 5-10 children per million each year [6]. ESRD is very rare but an important risk factor for pediatric mortality and morbidity. According to a study performed in Australia and New Zealand, age-specific mortality of pediatric ESRD patients was about 30 times higher than that of children without ESRD [6]. Either dialysis or KT as renal replacement therapy is required for survival of ESRD patients. Peritoneal dialysis is widely used as a renal replacement therapy for pediatric ESRD patients because the catheter is easy to install and maintain and has no technical problems regarding patient size [10-12]. In contrast, hemodialysis is hemodynamically more unstable than peritoneal dialysis in pediatric ESRD patients, and it is difficult to secure adequate blood vessels and blood volume in pediatric patients compared with adults [13,14]. Most pediatric ESRD patients using peritoneal dialysis or hemodialysis can survive, but the ideal treatment is KT because it is the best way to ensure a normal life and normal physical and mental development [3,4,6]. As previously reported, dialysis is associated with a risk of death that is four times higher than the risk associated with KT [6].
Growth failure is a crucial problem for pediatric ERSD patients and the largest concern compared with adult ESRD patients. One study noted that growth failure is associated with a more complicated clinical course and increased risk of death for pediatric ESRD patients [15]. Malnutrition, salt wasting, acidosis, secondary hyperparathyroidism, osteodystrophy, anemia, and resistance to growth hormones have been shown to be significant determinants of growth failure in pediatric ESRD patients [10,11,16]. Therefore, current clinical recommendations to achieve normal growth velocity in pediatric ESRD patients include adequate nutrition; control of renal osteodystrophy, anemia, and acid base and sodium balance; use of recombinant human growth hormone; and optimization of dialysis [11,15,17]. Our study showed that children who underwent KT before 10 years of age have significantly better growth rate changes per month than children who underwent KT after 10 years of age (0.15± 0.2% vs -0.05±0.17%, P=0.02). This result suggests that KT should be performed earlier to ensure better growth in pediatric ESRD patients, similarly to the report of the North American Pediatric Renal Transplant Cooperative Study (NAPRTCS) [3]. There were no patients who were treated with recombinant human growth hormone in our study. In conclusion, early KT can overcome growth delays in and return pediatric ESRD patients to a normal life.
In our study, there were 50 living-related donors (83.3%) and 10 deceased donors (16.7%) (Table 1). Among the livingrelated donors, 47 were the parents and three were others. No significant difference was noted between the two groups in the patient or graft survival analysis (Table 3). There are more living donors than deceased donors in Korea, and the living donors in cases of pediatric KT are mostly their family members [8,18]. This is different from other countries, including the Unites States, in which there are more deceased donors than living donors [19-21]. In 2015, the number of KT performed involving deceased donors in the United States was 38.5 per million population compared to 17.91 per million population in Korea. This shows that Korea has not only fewer total donors but also significantly fewer deceased donors, leading to a chronic organ shortage in Korea.
According to the report of NAPRTCS from 1987 to 2010 [3], the 5 year patient survival rates of living donor recipient under 18 years was 96.1% and among deceased donor recipients, the 5 year patient survival rates was 93.3%. In our study, the 5 year patient survival rates of living donor recipient and deceased donor recipient were 95% and 100%, respectively. Although the number of patients of our study is relatively small, we obtained similar results of the 5 year patient survival rates compared with NAPRTCS.
The graft survival rates of living donor recipients at 1, 3 and 5 years after KT in NAPRTCS from 1987 to 1990 were 89.4%, 81.1%, and 74.6, while 96.5%, 91.5%, and 84.3% from 2003 to 2010. In addition, the graft survival rates of deceased donor recipients at 1, 3 and 5 years in NAPRTCS from 1987 to 1990 were 75.1%, 63.4%, and 54.8, while 95.1%, 84.1%, and 78.0% from 2003 to 2010 [3]. In our study, the graft survival rates of total recipients at 1, 3, and 5 years after KT were 93%, 86%, and 68%. The graft survival rates of deceased donor recipients were 100%, 89%, and 59% and those of living donor recipients 91%, 85%, and 70%. Compared with the results of NAPRTCS, the graft survival rate of our study tended to be higher than those of NAPRTCS before 1990, but slightly lower than those after 2000. According to a study reported in Korea in 2000, the 5 years graft survival rate of Korean adult patients was about 88% [22].
As recently reported, ABO-incompatible KT might be an alternative solution for the potential living-related donor shortage [23-25]. In the past, KT was only possible with ABO-compatible KT, while ABO-incompatibility was considered a contraindication of KT. However, with the development of immunosuppressants since 2000, outcomes of ABO-incompatible KT were improved [24]. According to a report in Japan, outcomes of pediatric ABO-incompatible KT are equivalent to those of ABO-compatible controls [26].
In Korea, the first KT for an adult ESRD patient was performed in 1969; since then, the number of KT performed has been steadily increasing. The total number of KT performed in Korea in 2015 was 1891 (living donor, 990; deceased donor, 901) according to the 2015 annual transplant report by the Korean Network for Organ Sharing (KONOS). In contrast, only 39 KT were performed in pediatric ESRD patients <19 years old in 2015 in Korea. A total of 16,011 ESRD patients including 94 pediatric patients were waitlisted for KT at the end of 2015. The mean waiting time of pediatric ESRD patients for KT is 820 days for 1-5-yearolds, 392 days for 6-10-year-olds, and 1001 days for 11-18- year-olds, similar to that of adults. According to a paper published in 2012, in the United States, median time for wait-listing to KT in pediatric ESRD patients is 132 days (range, 49-304 days) [20].
In October 2005, the United Network for Organ Sharing implemented a Share 35 policy in which renal allografts from young deceased donors <35 years of age should be offered to pediatric ESRD patients <18 years of age. This policy has increased the number of pediatric KT from young deceased donors, meaning that pediatric ESRD patients were provided high-quality donors and a shortened wait time [20,21,27]. In Korea, the first KT from deceased donors was performed in 1979. In February 2000, the law on organ transplantation from deceased donors was enacted; since then, KONOS has actively implemented organ transplantation from deceased donors [24,28]. However, the supply of allograft kidneys from deceased donors remains insufficient. In Korea, living-related donor is the most common (usually the patient’s parents), but it cannot meet the demand. Therefore, active organ donation of deceased donors and living unrelated donors is needed for us to solve chronic shortage of organ transplantation. We also need a policy approach to pediatric KT because early KT provides good results for growth and development in pediatric ESRD patients.
Our study has several limitations. First, it was difficult to determine statistical significance due to the relatively small sample size compared to general adult subject studies. Second, its retrospective study design did not reflect improvements in surgical techniques, immunosuppressive agents, and long-term patient care over time. However, our study was conducted over a 30-year period and included a considerable number of patients from a single regional center in Korea.
In conclusion, our study findings imply that the earlier KT is performed, the better the growth and development outcomes of pediatric ESRD patients. Additionally, further analyses and monitoring of the clinical processes after KT are necessary and will contribute to decreased mortality rates and increased appropriate growth and development rates in pediatric ESRD patients.
Notes
Conflict of interest
No potential conflict of interest relevant to this article was reported.