Novel SLC5A2 Mutations and Genetic Characterization in Korean Patients with Familial Renal Glucosuria
Article information
Abstract
Purpose
Familial renal glucosuria (FRG, OMIM #233100) is a rare but relatively benign genetic condition characterized by persistent isolated glucosuria with a normal blood glucose level. We report three additional SLC5A2 mutations and examine their phenotypic and genetic characteristics in a Korean FRG cohort. We also reviewed the literature and summarized the genotypes of all Korean patients with FRG.
Methods
A genetic analysis was conducted by directly sequencing all 14 exons of the SLC5A2 gene and their flanking regions in six unrelated Korean children with FRG and their family members. Novel non-synonymous single-nucleotide polymorphisms were identified and compared with known mutations that are repeatedly detected in the Korean population.
Results
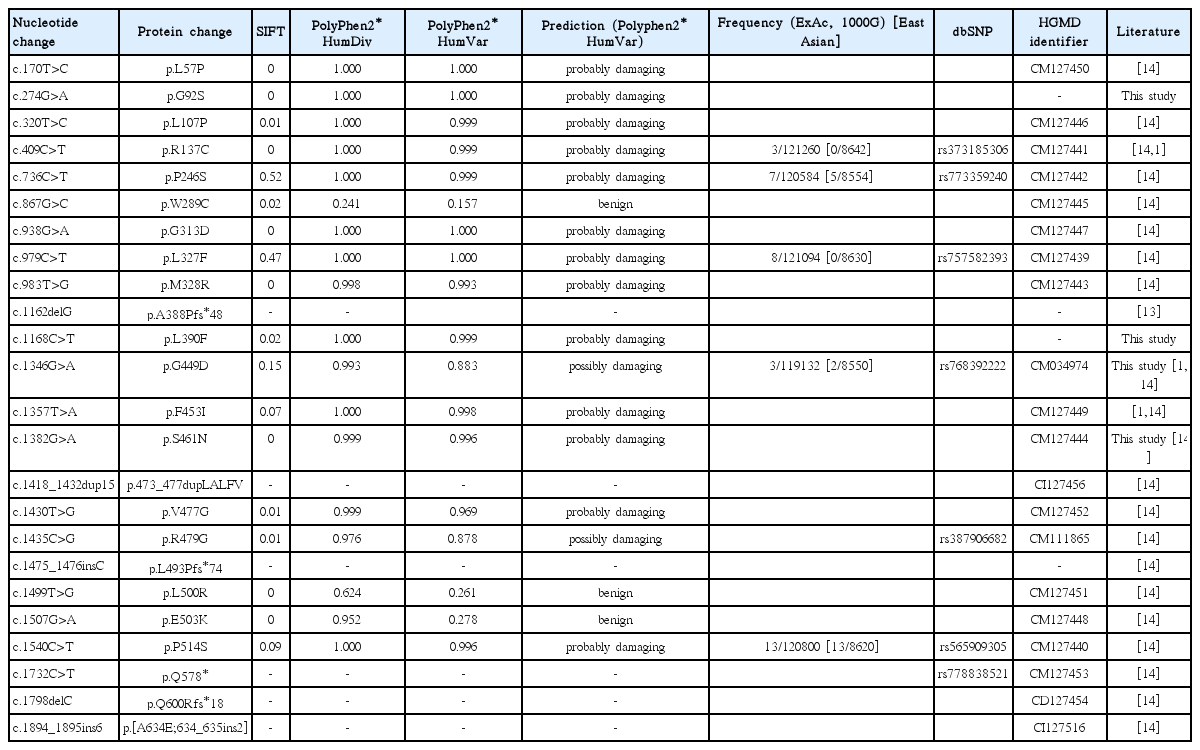
We found two novel mutations [c.274G>A (G92S) and c.1168C>T (L390F)] and one known [c.1382G>A (S461N)] mutation in each family and one recurrent mutation [c.1346G>A (G449D) (rs768392222)] in two pedigrees. The recurrent G449D was predicted to be “possibly damaging,” with a score of 0.883 in Polyphen-2, while G92S, L390F, and S461N were predicted to be “probably damaging,” with scores of 1.000, 0.999, and 0.996, respectively.
Conclusions
Two novel, one previously reported, and one recurrent mutation were identified in six Korean FRG pedigrees as causative mutations of renal glucosuria. Sequence variations in the SLC5A2 gene were frequently detected in children with persistent isolated glucosuria. A long-term follow-up of this FRG cohort is needed to understand how these specific SGLT2 mutations impair kidney function and energy homeostasis.
Introduction
Familial renal glucosuria (FRG, OMIM #233100) is a rare genetic condition characterized by persistent isolated glucosuria with a normal glucose level in blood and a relatively benign condition [1,2]. Mutations in the SLC5A2 gene that encodes glucose transporter in the renal proximal tubule are known to be a cause of this genetic condition and these mutations have been reported to be distributed across the entire gene region without a hot spot [3,4]. The lower affinity, high-capacity sodium-glucose cotransporter 2 (SGLT2), located in the early proximal convoluted tubule segment S1, plays a major role in the glucose reabsorption mechanism [5-7]. SGLT2 enables glucose homeostasis by reabsorbing the filtered glucose (approximately 180 g/day) and excreting glucose <0.5 g/day in adults [6,7]. The SLC5A2 gene is mapped to 16p11.2 and has 14 exons encoding 672 amino acids [8,9]. The degree of glucosuria depends on the mutation site, type and zygosity, so the clinical course varies according to the affected individual. Therefore, the codominance mode of inheritance with variable penetrance has been suggested [1,2]. Here, we added six patients to the Korean FRG cohort, reporting two additional novel SLC5A2 mutations and reviewed the genetic characteristics in this study and the literature.
Materials and methods
1. Study population
Blood samples from six Korean children and their families with isolated glucosuria were collected from May 2014 to June 2016 for the SLC5A2 mutation analysis. Written informed consent was obtained from all subjects and their family members for the molecular diagnostic tests whenever possible. This study was approved by the Institutional Review Board of Busan Paik Hospital and conducted according to the guidelines of the Declaration of Helsinki.
Five probands and one child were incidentally found to have glucosuria during a school health examination survey and a fever workup, respectively. Their mean age (four males and two females) was 10.8 years (range, 7-14 years). Diagnostic criteria were: (1) total 24-hour urine glucose excretion >0.5 g/1.73 m2; (2) normal glucose metabolism; 3) normal renal function without other pathological signs, such as hematuria, proteinuria, hyperphosphaturia, hypercalciuria, or metabolic acidosis.
2. SLC5A2 mutation analysis
Human genomic DNA was prepared from peripheral blood samples using the QIAamp DNA Blood Mini Kit (Qiagen, Hilden, Germany). A genetic analysis was conducted by directly sequencing all 14 exons of the SLC5A2 gene and their flanking regions in the six unrelated Korean children with FRG and their family members using the ABI 3130 Genetic analyzer (Applied Biosystems, Foster City, CA, USA) with the BigDye 3.1 Sequencing Kit (Applied Biosystems). Novel non-synonymous, single-nucleotide polymorphisms were identified and compared with the G449D recurrently detected in the Korean population. When an identical mutation has been reported separately over three times, we consider it as a recurrent (non-random) mutation.
3. Literature review and in silico prediction of altered protein function
A comprehensive review of the literature and a mutation database (Human Gene Mutation Database, http//www.hgmd.org) was conducted regarding the SLC5A2 sequence variations in FRG. To predict the deleterious effect of sequence variations, the substitutions found in all sequence variations detected in the Korean FRG cohort in this study and the literature were submitted to the web-based programs Sorting Intolerant From Tolerant (http://sift.jcvi.org) and Polyphen-2 (http://genetics.bwh.harvard.edu/pph2) and analyzed.
Results
1. Phenotypic analysis
None of probands in the FRG family had symptoms or signs of diabetes mellitus. No abnormal results were observed in the biochemistry, electrolytes, or a urinalysis, except glucosuria. No growth disturbances, polydipsia, polyuria, or dehydration were detected. Table 1 lists the clinical characteristics of these probands. No aminoaciduria was detected in the tested probands (patients 2, 3, and 4).
2. SLC5A2 mutation and in silico analyses
We found two novel mutations (c.274G>A (G92S) and c.1168C>T (L390F)) and one known mutation (c.1382G>A (S461N)) mutation in each family and one recurrent mutation (c.1346G>A (G449D) (rs768392222)) in two pedigrees (Fig. 1). The two novel variants were not found in any variation databases, including dbSNP, ExAc, and 1000G, or the literature (Table 2). One pedigree revealed no mutation in the coding sequence or its flanking regions. The recurrent G449D was predicted to be ‘possibly damaging’ with a score of 0.883 in Polyphen-2, while the novel G92S and L390F and the known S461N were predicted to be ‘probably damaging’ with scores of 1.000, 0.999, and 0.996, respectively.

Pedigrees with renal glycosuria and representative electropherograms of SLC5A2 mutation sites in this study. Dashed lines denote locations of the SLC5A2 gene mutations. PolyPhen-2 Human Var scores are displayed with heatmap charts on the right side of each electropherogram.
Discussion
In this study, sequence variations were detected frequently (83%, 5/6) by mutation analysis of the SLC5A2 gene in six unrelated families with persistent isolated glucosuria. Two novel (G92S and L390F), one known (S461N), and one recurrent (G449D) mutation were identified as causative mutations of renal glucosuria. Two novel and one known sequence variations were considered probably damaging, with a higher impact score in the prediction tools, compared with that of the known recurrent mutation. Despite a thorough evaluation of one pedigree with glucosuria, no mutations were detected in the entire coding region or adjacent intronic segments. A heterozygous large deletion or unknown mutation may be present in the other associated gene epigenetic alterations. In previous studies, wild type individuals also had the FRG pedigree [1,8].
To date, over 70 different SLC5A2 gene mutations have been reported and most are missense mutations and are suggested to be private (restricted to a single individual or pedigree) [3,10-14]. However, some of the mutations, such as IVS7+5G>A (c.885+5G>A), G449D (rs768392222), and P246S (rs773359240), have been reported recurrently [3,12,14]. In a study with a cohort of 23 Korean children with FRG, 19 mutations were detected, which were also private or novel, except for the recurrent G449D in seven pedigrees [14]. The present study reconfirms that most of SLC5A2 gene mutations are private and that G449D is a recurrent mutation in Korean FRG. IVS7+5G>A has been reported with the highest frequency in other ethnic groups [1,15]. Zhao et al. reported a 22-bp deletion in intron 7 (c.886(-10_-31)del), which was suggested to be the second high-frequency mutation (eight Chinese families) following IVS7+5G>A; thus, they concluded that this high frequency deletion in intron 7 should be screened in more patients with FRG and uniallelic or negative SLC5A2 mutations [9]. Although we reanalyzed the flanking regions of intron 7, we could not find any mutations in our FRG cohort.
FRG is generally an asymptomatic disorder except for glucosuria. Furthermore, glucosuria has been reported to be mild in most patients with FRG. However, some cases, especially those with homozygous or compound heterozygous mutations, have heavy glucosuria [1]. In those cases, severe glucosuria can be accompanied by generalized aminoaciduria [16]. Hyperuricosuria and hypercalciuria can also be secondary signs of FRG [17-19]. In this study, only one of six patients with FRG showed severe glucosuria, but aminoaciduria, hyperuricosuria, or hypercalciuria was not present. One patient with the recurrent G449D mutation showed severe glucosuria, whereas another patient with the same mutation showed only mild glucosuria, indicating the variable expressivity of this genetic disorder.
A new class of anti-hyperglycemic drug targeting the SGLT2 has been introduced to treat type 2 diabetes. This new class of anti-hyperglycemic drug has several advantages to deliver a beneficial effect on body weight and blood pressure. Use of SGLT2 inhibitors to diabetes mimics the beneficial effect of dysfunctional SGLT2 in FRG individuals. SGLT2 inhibitors can cause the same kinds of complications such as dehydration and urinary infections, which have been also reported in the severe form of FRG [3,20]. Inhibition or dysfunction of SGLT2 has pleiotropic effects on renal physiology [7,21,22]. The long-term outcome of patients with FRG is excellent, but the beneficial effect on metabolic homeostasis is unclear [11,23,24]. Thus, a long-term follow-up is needed to elucidate the life-long consequences of inhibiting or altering SGLT2. In addition, the mutation profiles of SLC5A2 gene could provide useful clues for the protein function and new drug targets.
In summary, we identified five mutations, including two novel, one known, and one recurrent missense mutation, in six Korean FRG pedigrees. This study expands the knowledge about the genetic characteristics of SLC5A2 in Korean FRG. A long-term follow-up of this FRG cohort is needed to understand how these specific SGLT2 mutations impair kidney function and energy homeostasis and to predict which domains would be targets of SGLT2 inhibitors.
Notes
Conflict of interest
No potential conflict of interest relevant to this article was reported.