Case report
An 8-year-old male patient visited the emergency department in 1994, with skin rashes on both legs, abdominal pain, and arthralgia occurred 4 days ago. He received conservative treatment at a local clinic for 2 days but showed no improvement. The patient had neither recent medical history nor family history of HSP. Vital signs were measured as: blood pressure, 100/60 mmHg; pulse rate, 92 beats/min; respiratory rate, 22/min; and body temperature, 37.0ã. On physical examination, bilateral tonsillar enlargements, numerous palpable purpura in both legs and buttocks, and knee joint tenderness and abdominal tenderness were noted, but there was no rebound tenderness.
The laboratory test revealed the following results: white blood cell count, 13,300/mm3; hemoglobin, 13.3 g/dL; platelet count, 372,000/mm3; erythrocyte sedimentation rate, 18 mm/hr; serum total protein, 5.7 g/dL; albumin, 3.4 g/dL; blood urea nitrogen(BUN), 15.1 mg/dL; creatinine, 0.9 mg/dL; C-reactive protein, 1.2 mg/dL; antistreptolysin O (ASO), 1,317 IU/mL; prothrombin time, 12.8 seconds; and activated partial thromboplastin time, 27 seconds. Urinalysis showed 1+ protein and 2+ blood. Creatinine clearance (Ccr) calculated through a 24-hour urine collection was 145.3 ml/min/1.73m2.
The patient was diagnosed with HSP and treated with intravenous dexamethasone (0.1 mg/kg per dose every 6 hours) from the 1
st day of hospitalization. The patientãs conditions were stable with intermittent abdominal pains for the 1
st week of hospitalization. On the 8
th day of hospitalization, he developed a sudden severe abdominal pain with the expansion of purpura on both legs and feet including buttocks, and abdominal ultrasonography showed extensive small intestinal wall thickenings and minimal ascites (
Fig. 1). On the 10th day of hospitalization, patientãs abdominal pain and bloody diarrhea worsened, but no sub-diaphragmatic air was found on simple X-ray. On the same day, Technetium-99m-labeled red blood cell scan (
99mTc-RBC scan) revealed diffuse intramural and intraluminal hemorrhages, but no extraluminal bleeding to abdominal cavity (
Fig. 2). On the 12
th day of hospitalization, abdominal distension developed and serum hemoglobin decreased from 11.6 g/dL to 9.2 g/dL. Under the suspicion of intestinal perforation, paracentesis was performed and 150 cc of serosanguinous fluid was drained to confirm hemoperitoneum.
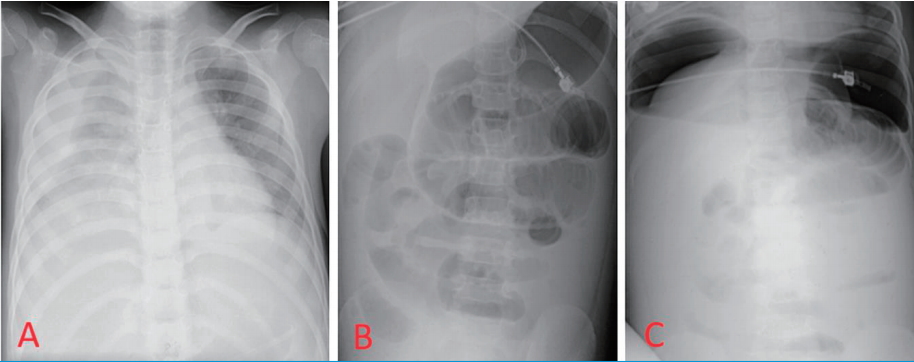
On the 13
th day of hospitalization, the patient complained of dyspnea. Pleural effusion was found on chest X-ray, and chest tube drained 200 mL of bloody fluid. Direct and rebound tenderness were present on the entire abdomen area, and bilateral sub-diaphragmatic free air was detected on simple X-ray (
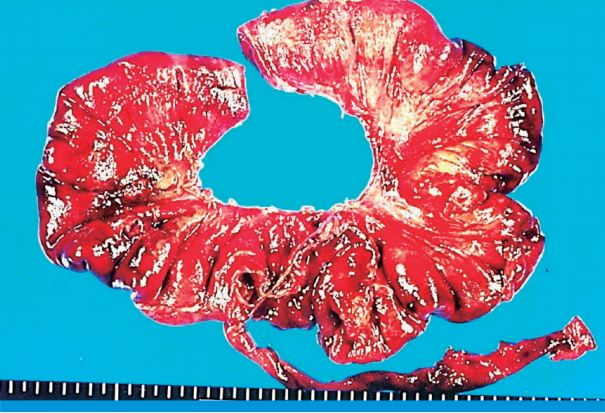
Fig. 3). The patient was referred to pediatric surgery and emergency laparotomy was done to reveal a large amount of pus collection in the peritoneal cavity. Multiple linear perforations were noted along the antimesenteric border at the 25 cm proximal portion of ileum from the ileocecal valve. About 50 cm of small intestine was necrotized, and segmental resection was performed to reveal hemorrhagic patches on the intestinal membrane and ischemic necrosis (
Fig. 4). In the mesentery, multiple small arterial hemorrhagic points were noted which might have contributed to intestinal necrosis and perforation.
After the operation, the patient received intravenous antibiotics for 2 weeks and then the skipped dexamethasone was restarted as oral prednisolone (1 mg/kg/day divided into 3 doses). The patient tolerated well and recovered gradually, despite persistent purpuras, intermittent abdominal pains and urinary abnormalities. The patient was discharged on the 28th postoperative day.
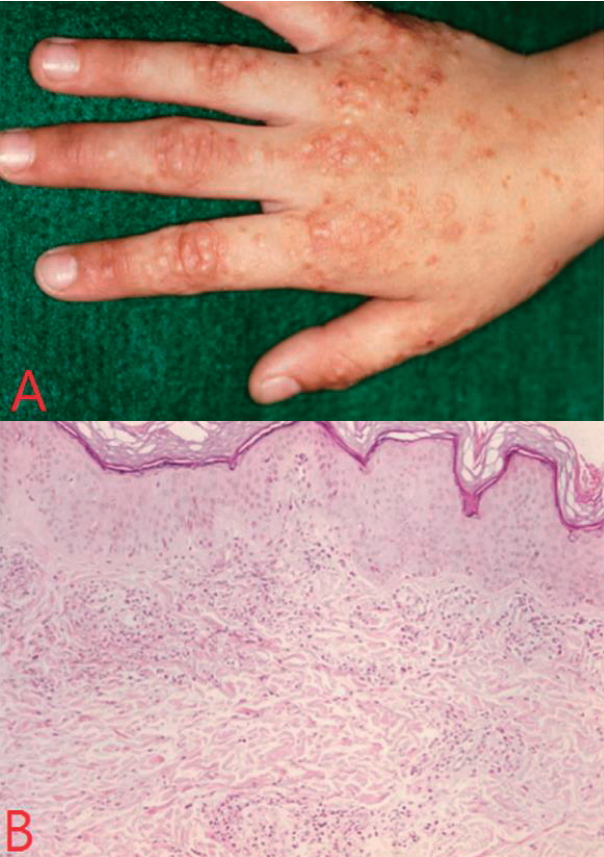
One month after discharge, new itching nodular skin rashes on extensor surface of hands, fingers, and elbows began to develop and became more severe and skin biopsy of the hand lesion on 56
th day of discharge, revealed leukoclastic vasculitis (
Fig. 5).
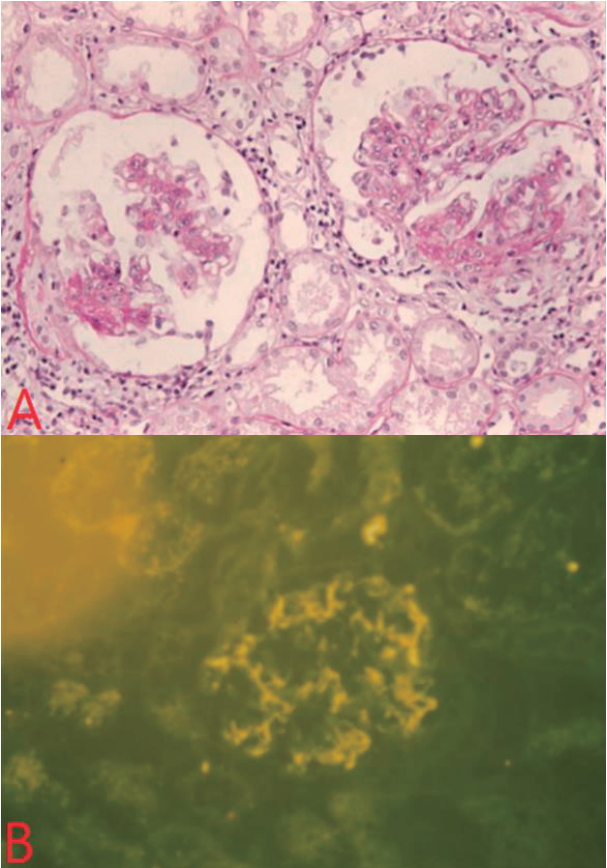
Urinary abnormality, microscopic hematuria (5ã30 RBC/HPF) and proteinuria (30ã100 mg/dL) continued for 2 months after the discharge despite oral prednisolone and angiotensin-converting enzyme inhibitors (ACEis) medications, and kidney biopsy on the 62
nd discharge day revealed mesangial cell proliferation, and increase in mesangial matrix with immunoglobulin A deposition on immunofluorescence study (
Fig. 6). Cyclosporine (5 mg/kg/day oral in two divided doses) and ACEi (5 mg daily) were given for 12 months. Proteinuria resolved after 6 months of treatment, and hematuria, after 2 years.
And on the follow-up after 25 years, the patient visited our hospital for medical check-up at the age of 33 years old. After examinations, he showed normal physical and renal status without any gastrointestinal signs; height 179 cm, body weight 72 kg, BUN 24.4 mg/dL, serum creatinine 1.20 mg/dL, and CCr 108 ml/min/1.73m2 by 24-hour urine collection, and there was no urine abnormality.
Discussion
HSP is a systemic vasculitis caused by IgA-containing immune complexes depositing in small vessel walls, such as arterioles and venules, which activates the complement system [
1]. It is known that HSP occurs in 20 cases per 100,000 children annually, and found twice as many in males than females [
4]. Peak age incidence is 4ã6 years, but can occur in all age groups [
4,
8]. Upper respiratory infection such as streptococcus, staphylococcus, and parainfluenza or vaccination, medication such as antibiotics, and autoimmune disease are often preceded [
6]. In this patient, serum ASO level increased to 1,315 IU/mL with tonsillar hypertrophy, suggesting a precedence of streptococcal infection.
European League Against Rheumatism/Pediatric Rheumatology European Society (EULAR/PReS) defined the diagnostic criteria of HSP as follows: palpable purpura in the presence of at least one of the following four feature: diffuse abdominal pain, arthritis or arthralgia, any biopsy showing predominant IgA deposition, and renal involvement (any hematuria and/or proteinuria) [
5]. Skin involvement is the most common manifestations of all HSP patients [
1,
7]. Petechiae and palpable purpura are common forms, but it may appear as erythema, bullous, or nodular lesions [
7]. It is characteristically distributing mainly on bilateral lower limbs but may also appear in areas such as face, ears, and hands [
7]. In our case, nodular skin lesions appeared newly on the dorsum of the hands and elbows two months after the onset of the disease. On the 84
th day of disease onset, skin biopsy was done and showed leukoclastic vasculitis.
Renal involvement is considered upon presence of hematuria [
1]. Proteinuria is very rarely found alone without hematuria [
1]. Nephritis develops within 3 months of skin manifestations and mostly resolves within 1 year, but consistent hematuria and proteinuria may progress to end-stage renal disease (ESRD) [
7]. Renal involvement is the most crucial prognostic factor in HSP [
9]. In this patient, both hematuria and proteinuria occurred from the beginning of HSP and persisted more than three months. Proteinuria resolved after 6 months of cyclosporine therapy, and hematuria disappeared completely after 2 years.
Gastrointestinal involvement occurs in 50ã75% of HSP patients and in 10ã30%, it may precede the skin lesions [
2]. Symptoms such as abdominal pain, vomiting, hematemesis, and hematochezia can be found [
2]. Abdominal pain is the most common symptom and it may be colicky [
7,
9,
11]. It rarely accompanies tenderness or rebound tenderness and mostly improves within a few days with conservative treatment [
2]. If abdominal pain persists with abdominal distension or vomiting, close observation should be made to distinguish from intussusception, acute appendicitis, intestinal obstruction or perforation [
11].
Intestinal perforation is a rare complication of HSP. It occurs mainly in the small intestine, but some cases where multiple perforations involve stomach and duodenum have been reported [
2]. It is due to ulceration by vasculitis and consequent ischemic changes and necrosis in the bowel wall [
2].
It is known that severe abdominal manifestations may increase the risk of renal damage in HSP patients [
12]. We also had experienced such a case [
13] before, but to the contrary, this patient resulted in normal renal function and normal urine findings on the follow-up examinations after 25 years.
HSP improves simply by conservative management in most cases but the treatment of HSP still remains controversial [
10,
14]. The nationwide study reported 56.6% of children with HSP were prescribed steroids in Korea [
10]. The recent Cochrane review concluded that there is no evidence of benefit for the use of steroid to prevent kidney disease in children with HSP [
14]. But Jauhola et al. reported prednisone treatment reduced the severity of abdominal or joint pain and also the durations of abdominal pain [
15]. In this case, despite steroid treatment the gastrointestinal symptoms became more severe and led to intestinal necrosis and perforation, requiring surgical resection of small bowel on the 13
th day of hospitalization (
Fig. 4).
Pulmonary hemorrhage is an unusual complication of HSP [
16]. A systematic review reported the prevalence of pulmonary complication ranged 0.8% to 5%, including hemoptysis, dyspnea, and chest infiltration [
16]. In this case, the patient complained dyspnea and showed pleural fluid collection on the right side and chest tube drainage revealed serosanguinous pleural effusion, suggesting bleeding from pleural cavity (
Fig. 3).
In conclusion, we report a case of HSP with multiple complications such as intestinal perforation, pulmonary hemorrhage and various skin rashes, nephritis and its longterm outcome.


 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link XML Download
XML Download Full text via DOI
Full text via DOI Download Citation
Download Citation
 Print
Print