Introduction
Pediatric kidney transplantation (KT) is the treatment of choice for children with end-stage renal disease (ESRD). Annual cases of pediatric KT have steadily increased up to 70 cases per year in South Korea [
1]. The renewed kidney allocation system, which has been valid since 2018, prioritizes children younger than 19 years on the transplant waitlist for pediatric cadaveric donations. With the longer life expectancy and graft survival granted by advances in immunosuppressant therapy, long-term complications associated with significant morbidity and mortality, such as malignancies, are becoming more of a concern for pediatric transplantation recipients [
2]. Malignancies occurring after KT result from various complex factors, including the immunological condition of the recipient, immunosuppressive treatment, oncogenic viruses, and other possible synergistic effects [
3]. The epidemiological pattern of malignancies among pediatric KT recipients is known to differ from that among adult KT recipients and the general population. While malignant lymphoma and non-melanoma skin cancer, thyroid cancer, stomach cancer have been identified as common post-transplant malignancies (PTMs) among adults, including at our center [
4,
5], post-transplantation lymphoproliferative disease (PTLD) and squamous cell carcinomas or basal cell carcinomas of the skin are commonly reported PTMs among pediatric patients after long-term follow-up [
3,
6]. PTLD is known to be associated with Epstein-Barr virus (EBV) infection, either primary infection or reactivation due to immunosuppression [
7]. Risk factors for PTLD include EBV-naïve recipients with EBV-positive donors, younger age at transplantation, more aggressive immunosuppression; additionally, PTLDs are most likely to arise within the first year after transplantation [
8,
9]. To evaluate incidence, malignancy types, manifestations, and outcomes, we analyzed our single-center experience of long-term follow-up of pediatric KT recipients.
Results
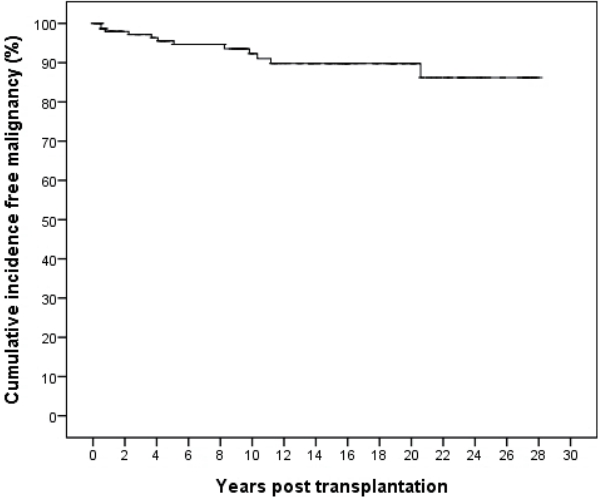
A total of 155 patients were included in this study who received KT during the study period (Male:Female=95:60). The KT recipients had a mean age of 13.2┬▒4.2 years (median 14.3, range 1.4ŌĆō18.0 years). The mean follow-up period was 11.5┬▒8.3 years (median 9.6, range 0.1ŌĆō28.1 years). In total, 12 recipients (7.7%) were diagnosed with a malignancy after KT (Male:Female=6:6). The mean age at cancer diagnosis was 19.4┬▒7.4 years. These patients underwent KT at a mean age of 13.0┬▒4.0 (median 12.7, range 4.4ŌĆō18.0) years. The mean interval between KT and malignancy diagnosis was 6.4┬▒5.9 (median 4.6, range 0.5ŌĆō20.6) years. The overall cumulative incidence of malignancy was 13.8 %, with a cumulative incidence of 7.7% at 10 years post-KT, 10.2% at 15ŌĆō20 years post-KT (
Fig. 1). Of the 12 patients diagnosed with PTMs, three (25%; all with PTLD) were diagnosed with their PTMs within the first year post-KT.
Four types of cancer were diagnosed among the 12 PTM patients: nine patients with PTLD (75.0%, Male:Female=4:5), one patient with T-cell acute lymphocytic leukemia (ALL) (8.3 %), one patient with mucoepidermoid cancer of the hard palate, and the one patient with papillary thyroid cancer. With an overall incidence of 5.8% (9/155), PTLD was diagnosed within a mean of 3.7┬▒3.4 years (median 3.7, range 0.5ŌĆō9.8 years) after KT, which was a shorter mean interval than other malignancies (mean 14.2, median 11.2, range 10.3ŌĆō20.6 years). Characteristics of the patients with PTM are summarized in
Table 1, and the clinical course of each patient is described in
Table 2.
Seven (58.3%) of 12 PTM patients received kidneys from cadaveric donors. The incidence of PTM among all cadaveric KT recipients was 15.6% (7/45). Five PTM patients (41.7%) underwent living donor KT. The incidence of PTM among living donor KT recipients was 4.5% (5/110) (
Table 1). The immunosuppressant regimens at the time of diagnosis are described in
Table 1 and
Table 2.
Recipient and donor EBV serology data at the time of KT are described in
Table 1. For the nine patients diagnosed with PTLD, primary EBV infection occurred at the time of PTLD diagnosis in three patients. Four patients who had prior EBV exposure underwent conversion of serum EBV DNA titer from negative to positive at the time of diagnosis, and the remaining two patients with prior EBV exposure had a negative serum EBV DNA titer at the time of diagnosis. Three patients with primary EBV infection after KT were diagnosed with PTLD within 9 months after KT, showing an earlier onset than the other patients with PTLD (
Table 2).
The first intervention for all PTM patients after their PTM diagnosis of was to moderate their immunosuppression (discontinue antimetabolite agents [AZT or MMF] and/or lower the CNI dosage) or convert their maintenance immunosuppression medications of CNI to sirolimus. Seven patients (six patients with PTLD and one patient with thyroid cancer) were changed from tacrolimus to sirolimus. The patients diagnosed with PTLD and T-cell ALL all underwent chemotherapy according to the extent of disease and pathologic diagnosis. Patient 12, with a mucoepidermoid tumor, underwent an excision operation followed by adjuvant chemotherapy, and Patient 10 with PTC underwent surgical resection of thyroid cancer with cervical lymph node dissection.
The overall mortality rate among the 12 PTM patients was 33.3% (4/12). Among the nine patients diagnosed with PTLD, three patients (33.3%) died after disease progression (Patient 8) or infection during chemotherapy (Patient 7 and Patient 9). Five patients were cured without recurrence, and one patient, at the time that this article was composed, was still on chemotherapy for residual lesions. Patient 12, with mucoepidermoid cancer of the hard palate, also died after disease progression despite surgical resection and chemotherapy. The patients with thyroid cancer and T-cell ALL were all cured without recurrence. All patients who survived did not show deterioration of renal function during PTM treatment. However, during follow-up, four (44.9%) of nine PTM survivors had preserved renal function, and two patients had deteriorated renal function, with a median GFR of 34.5 ml/min/1.73 m2 (range 30ŌĆō39 ml/min/1.73 m2). The other three patients progressed to ESRD after a median of 33 months (range, 24ŌĆō70 months) after PTM remission. At the time of composition of this article, two patients diagnosed with PTLD and thyroid cancer were on renal replacement therapy, and the patient with T-cell ALL had progressed to ESRD and were being considered for renal replacement therapy.
Discussion
PTMs have a significant impact on the morbidity and mortality of pediatric KT recipients. In this study, we evaluated the incidence of PTM over long-term follow-up and assessed the clinical course and factors associated with PTM development.
The overall incidence of PTM and overall incidence of PTLD in this cohort are comparable with previous studies, which have reported incidences ranging from 7.3ŌĆō15.4% for all types of PTM associated with pediatric KT; however, the PTLD incidence at our center was higher than the overall incidence estimates of 2.2ŌĆō5.3% reported elsewhere [
3,
6,
12]. About half of the PTM cases occurred within 10 years after KT when analyzed according to the time to diagnosis (
Fig. 1), and this trend was in line with a previous study by Koukourginanni et al., who reported findings from 20 years of follow-up in France, including a cumulative incidence of 6.9% at 10 years and 10.2% at 15 years [
3]. However, Serrano et al. reported a higher incidence over a longer follow-up interval, showing cumulative PTM incidence values after pediatric KT in Minnesota of 4% at 10 years, 13% at 20 years, 26% at 30 years, and 36% at 40 years, which might be explained by the longer follow-up duration, which allowed them to detect and include the cases of adult-type epithelium-derived cancers [
6]. These studies suggest that surveillance for PTMs associated with pediatric KT, such as PTLD, should be emphasized, especially during the first 10 years post-KT, and adult-type cancers should be included within the differential diagnosis thereafter.
We found sexual difference in terms of incidence of PTM in this study. While more male patients received KT (Male: Female=95:60), incidence of PTM was higher in female (10 %, 6/60) than in male (6%, 6/95) which was accentuated in the incidence of PTLD (female 8%, male 4%). This higher incidence of PTM including PTLD in female was also noticed in the recent Korean study [
13], although the sex was not evaluated as a factor for risk of PTLD or donor type was stratified according to recipient sex in the study. Compared with previous studies showing higher incidence of PTM in male [
3], our results might be attributable to the higher rate of cadaveric donors in female PTM patients, or multifactorial effects including EBV serostatus, presence of acute rejection and possible sexual hormonal effects requiring elucidation .
The most frequently diagnosed PTM in our study was PTLD, which developed within a mean of 4.3┬▒3.3 years after KT at our center. PTMs other than PTLD were all diagnosed more than 10 years after KT. Patients with PTLD in our study underwent KT at younger age (mean 12.1 years, median 11 years) than the overall mean age of KT (13.2 years, median 14 years). The increased PTLD risk associated with a younger age at KT is mainly attributable to the frequent EBV seronegativity at the time of KT, and the predisposition to primary EBV and CMV infections in the early period after KT [
9,
14]. One-third of the PTLD patients in our study were EBV-naïve before KT and had primary EBV infection at the time of PTLD diagnosis; all nine of the PTLD patients were diagnosed within 9 months after KT. It is obvious from these findings that young age and EBV-naïve status are necessary but not always sufficient for PTLD development. However, primary-EBV-associated PTLD had a more rapid onset within the first year after KT than other cases, emphasizing the importance of surveillance of early PTLD, especially for EBV-naïve KT recipients. This was also stressed by Schober et al. presenting the distinct characteristics of early and late PTLD after transplantation: early PTLD within 3 years of transplantation is mainly an EBV-driven, extra-nodal disease caused by insufficient immunosurveillance, unlike late-onset disease [
15]. Nevertheless, the fact that all histological diagnoses from our patients were consistent with diffuse-large B-cell lymphoma with extra-nodal involvement (liver, spleen, brain, small bowel, bone, lung), implies that EBV plays a role in the development of PTLD by infecting and inducing malignant transformation of B-cells [
4,
13,
16].
Accumulating evidence suggests that it is not the choice of regimen but the intensity, especially the degree of T-cell immunosuppression, that is associated with the risk of PTLD [
9,
17]. This is associated with the impairment of EBV-specific T-cell-mediated immunity, which disrupts cytotoxic T-cells to check the EBV-infected B-cells and promote the development of PTLD. The magnitude of immunosuppression of patients with PTLD could not be objectively confirmed and compared in this study, but the tacrolimus drug level (and the cyclosporine level in one patient) was almost within the target range according to the time after KT, except for one patient who showed acute T-cell-mediated rejection with antibody-mediated rejection 11 months before the diagnosis of PTLD and was on a stronger immunosuppression regimen. Therefore, we could not fully verify the association between PTLD and the level of immunosuppression in this study. Other factors proposed to be associated with the development of PTLD, including complete HLA-DR mismatch, certain types recipient HLA, and host genetic variations, were not analyzed in this study and are yet to be further investigated in larger prospective studies [
12,
18-
20].
PTM treatment among our patients was adherent to the existing recommendations [
8]. Patients all discontinued maintenance antimetabolite agents (AZT or MMF), and CNI doses were reduced. Additional chemotherapy was applied for all PTLD patients, including rituximab with CHOP (cyclophosphamide, doxorubicin, vincristine, prednisone) as the fundamental regimen [
9]. Four of the six PTLD survivors, and all three patients with non-PTLD PTM changed from CNI to sirolimus while maintaining steroid therapy. Sirolimus, as a mammalian target of rapamycin (mTOR) inhibitor, has both immunosuppressive and antiproliferative effects, and has been shown to inhibit tumor growth and progression [
9]. Pascual et al. reported the remission of 19 patients with post-KT PTLD with the addition of proliferation signal inhibitors and minimizing CNIs, with or without concurrent chemotherapy [
21]. Among seven patients who used sirolimus after the diagnosis of PTM at our center, six patients were cured without recurrence, but Patient 8 experienced recurrence with progression. Although adverse events such as poor wound healing, oral ulceration, and proteinuria have been reported with sirolimus, no severe adverse events were reported among our patients, supporting the utility of mTOR inhibitors for PTM including PTLD.
The PTM-associated mortality rate was highest in association with PTLD than other malignancies in our study, and higher compared with previously reported mortality rates. Not only uncontrolled PTLD without complete remission despite immunosuppression reduction with concurrent chemotherapy, but complications, such as neutropenia and infection during chemotherapy, also contributed to mortality in our study. This was unlike the finding, reported by Jeong et al., of a 100% remission rate among PTLD patients for whom mortality was only associated with complications of infection, or the report, by McDonald et al., of 100% remission from a multicenter study with a mortality rate of 5.2% due to PTLD recurrence in one patient [
12,
22]. The impact of PTM on allograft preservation is still debated. Serrano et al. reported an increased hazard ratio of both death and allograft loss among PTM patients compared with patients without PTM, while Francis et al. reported that PTM development was only associated with mortality, not allograft loss [
6,
23]. Among our surviving PTM patients, allograft function was preserved during PTM treatment, even if the malignancy involved the allograft in one patient (Patient 1,
Table 2.). Although one patient with PTLD, and two patients with other PTMs eventually progressed to ESRD (3.25, 16.1, and 21.1 years after KT respectively) due to rejection or chronic allograft nephropathy, no allograft loss occurred during PTM treatment in our study.
Although the significance of this study comes from the long-term follow up evaluation at a single center, there were some limitations to consider. First, the retrospective observational nature of the study and the small sample size limits the generalizability of the results. Regarding the EBV serology, the timing of laboratory tests and the method of EBV load analysis might have changed over the course of the period under study, and this would have affected the detection of EBV, therefore preventing like-for-like comparisons for the entirety of the study period.
 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link XML Download
XML Download Full text via DOI
Full text via DOI Download Citation
Download Citation
 Print
Print