Clinical Outcomes of Non-carbapenem Treatment for Urinary Tract Infections Caused by Extended-spectrum β-lactamase-producing Escherichia coli
Article information
Abstract
Purpose
The purpose of this study was to investigate the clinical outcomes of non-carbapenem treatment for urinary tract infections (UTIs) caused by extended-spectrum β-lactamase (ESBL)-producing Escherichia coli (E. coli) in young children.
Methods
We retrospectively reviewed the medical records of children under 2 years of age who were diagnosed and treated for UTIs caused by ESBL-producing E. coli from September 2014 to March 2020.
Results
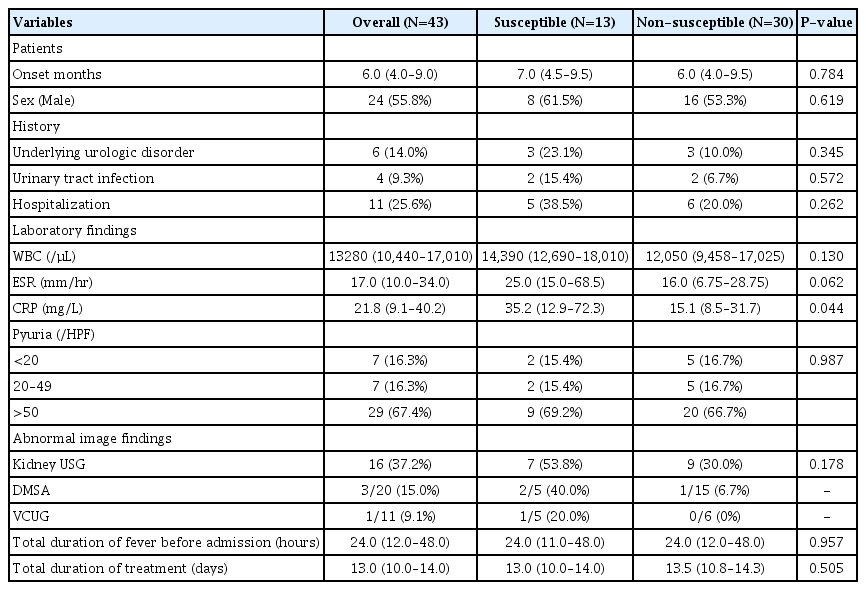
Forty-three children under 2 years of age were treated with non-carbapenem antimicrobials for UTIs caused by ESBL-producing E. coli without bloodstream infections. The overall clinical and microbiological success rates for empirical antimicrobial treatment were 90.7% and 97.7%. Three of the patients (7.0%) experienced a relapse of UTI within a month. An in vitro susceptibility test showed that two patients were sensitive and one was resistant to the antimicrobial treatments. Furthermore, there were no significant differences in the time to defervescence, clinical success, microbiological success, and relapse rate between the susceptible (n=13) and non-susceptible groups (n=30).
Conclusion
In this study, the overall relapse rate of patients treated with non-carbapenem antimicrobials was 7.0%. The patients showed high success rates in the clinical and microbiological responses to the non-carbapenems regardless of the results of the in vitro antimicrobial susceptibility test. These results provide evidence that non-carbapenems may be viable alternative treatments for UTIs caused by ESBL-producing E. coli.
Introduction
Urinary tract infections (UTIs) caused by extended-spectrum β-lactamase (ESBL)-producing organisms are increasingly becoming prevalent [1-5]. ESBL is an enzyme produced by Gram-negative bacterial species that hydrolyze most of the β-lactam antibiotics, including cephalosporin and penicillin [6-8]. Carbapenem is the most potent and broadest β-lactam antibiotics because it has an unsaturated bond between C2 and C3, and the carbon atom replaces the sulfur atom in the thiazolidine ring in the penicillin core structure. It is considered as the gold standard treatment for ESBL infections [9,10]. However, due to the possible emergence of carbapenem-resistant organisms, and a lack of therapeutic options in the event of carbapenem treatment failure, clinicians continue to raise concerns about the overuse of carbapenem and are contemplating over strategies for its selective use [11-15] .
Most clinicians experience improvement in clinical symptoms and the achievement of microbiological responses without the use of carbapenem in children with UTIs caused by ESBL-producing organisms. This phenomenon is difficult to explain clearly; however, the clinical and microbiological responses to antimicrobials used for UTIs and bloodstream infections are presumably different, since the urine drug concentration is generally higher than that achieved systemically [16-19]. As such, in vitro antimicrobial susceptibility test results in patients with UTIs caused by ESBL-producing organisms are not always helpful in predicting the recurrence of UTIs or kidney scarring. Since few studies have been reported on this issue in the pediatric population, insufficient evidence is available to support the use of non-carbapenem antimicrobials as therapeutic options for cases of uncomplicated UTIs caused by ESBLproducing organisms that are responsive to initially administered antimicrobials [17,20-23]. Therefore, clinicians keep questioning whether antimicrobials should be changed based on the results of in vitro antimicrobial susceptibility tests, despite clinical and microbiological success with the initial empirical antimicrobials. This study attempts to address the matter by investigating the clinical outcomes of non-carbapenem treatment for UTIs caused by ESBLproducing Escherichia coli (E. coli) in young children.
Materials and Methods
1. Study design and definitions
We reviewed the electronic medical records of individuals diagnosed with UTIs caused by ESBL-producing E. coli between September 2014 to March 2020 at Hallym University Kangnam Sacred Heart Hospital. All patients under 2 years of age with a single microbial infection confirmed by the isolation of ESBL-producing E. coli from urine samples and without bloodstream infections were included. All urine sample collections were obtained via urinary catheterization. Patients who met all three criteria below were considered to have UTI. The axillary body temperature measured in the hospital was >38℃ (100.4 °F), the number of bacterial colonies in urine culture was above 5×104/mL, and pyuria was greater than 5 white blood cells (WBCs) per high power field (HPF). The antimicrobial given to each patient was chosen after careful discussion with the patient’s parents about the available antimicrobials and their pros and cons. This study was approved by the institutional review board of Hallym University Kangnam Sacred Heart Hospital (IRB 2020-05-003).
The antimicrobial susceptibility test was performed with a disc diffusion test recommended by the Clinical and Laboratory Standards Institute guidelines [24]. Antimicrobial therapy administered before in vitro antimicrobial susceptibility test results were available was considered empirical, whereas antimicrobial therapy administered afterward was considered definitive. Patients who started effective empirical therapy or had subsequently switched to susceptible antimicrobials during the course of their infection were classified as “susceptible”, whereas patients who received non-susceptible antimicrobials for the treatment of their current UTI episode were classified as “nonsusceptible”. The main outcome variables were time to defervescence, clinical success, microbiological success, and relapse. Time to defervescence was defined as the time that patient maintain a body temperature below 38℃ (100.4 °F) without the help of antipyretics after the initiation of antimicrobials. Clinical success was defined as resolution of signs and symptoms within 3 days after the start of treatment. Microbiological success was defined as a negative urine culture within 3 days after the start of antimicrobial treatment. Patients who developed UTI caused by the same pathogen within a month after treatment initiation were considered relapse cases.
2. Statistical analyses
We used the median and interquartile range (IQR) to express continuous variables and percentages to express categorical variables. The Mann-Whitney test was used to evaluate differences in continuous variables, and chisquared test or Fisher’s exact test was used to evaluate differences in categorical variables between groups. P-value <0.05 was considered to be statistically significant. We used IBM SPSS Statistics for Windows, Version 26.0 (IBM Corp, Armonk, NY) for all analyses.
Results
1. Study population and baseline characteristics
During the study period, 43 children treated with noncarbapenem for UTIs caused by ESBL-producing E. coli were identified. Third-generation cephalosporin, cefotaxime, was the most commonly administered empirical antimicrobial agents (37/43, 86.0%), whereas in remaining cases, β-lactam/β-lactamase inhibitor (6/43, 14.0%), piperacillin/ tazobactam, was used. The antimicrobial agent was changed in 7 patients, from third-generation cephalosporin to β-lactam/β-lactamase inhibitor based on in vitro antimicrobial susceptibility test results (Fig. 1).
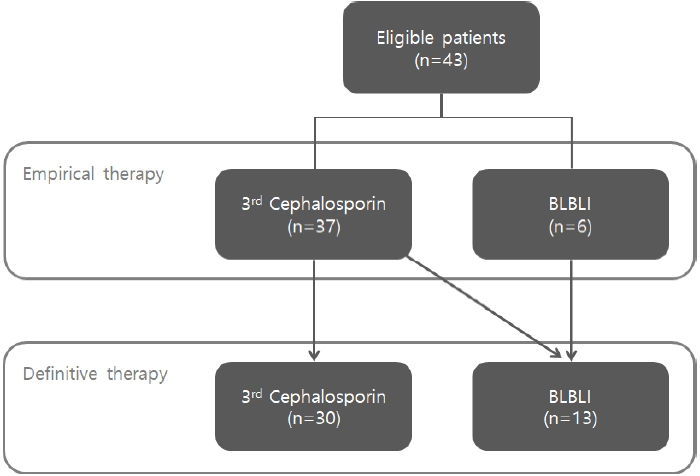
Flow chart of patients included in the study. 3rd Cephalosporin, third-generation cephalosporin; BLBLI, β-lactam/β-lactamase inhibitor.
The overall median age of patients was 6 months (IQR 4.0–9.0) and 24 patients were male (55.8%). With regards previous medical history, 6 patients (14.0%) had an underlying urologic disorder such as congenital hydronephrosis and mild pelviectasia, 4 patients (9.3%) had previously experienced UTIs, and 11 patients (25.6%) had been previously hospitalized. All patients underwent kidney ultrasonography (USG) and 16 patients (37.2%) showed abnormalities. Of the 20 patients who underwent the Tc-99m dimercaptosuccinic acid renal scan (DMSA) during the acute phase, 3 patients (15.0%) were found with cortical defects. Vesicoureteral reflux was found in 1 out of 11 patients (9.1%) who underwent voiding cystourethrography (VCUG). Since the number of patients who underwent DMSA and VCUG was small, statistical analysis could not be performed. In all patients, the strains isolated were sensitive to carbapenem and resistant to cefotaxime, a thirdgeneration cephalosporin. Of the strains isolated, amoxicillin- clavulanic acid were sensitive to 28 (65.1%) and piperacillin-tazobactam were sensitive to 41 (95.3%). The median total duration of fever before admission was 24 hours and the median total duration of treatment was 13 days (IQR 10.0–14.0) (Table 1).
2. Comparison between susceptible and nonsusceptible groups
As shown in Table 1, 30.2% of patients (13 of 43) were susceptible and 69.8% of patients (30 of 43) were non-susceptible to treated non-carbapenem antimicrobials. No statistically significant differences were found in the demographics, medical histories, total duration of fever before admission and total duration of treatment days between susceptible and non-susceptible groups. In laboratory test results, C-reactive protein level was higher in the susceptible group than in the non-susceptible group (P=0.044), and the levels of other inflammatory markers such as white blood cell count, erythrocyte sedimentation rate and pyuria were not significantly differ between the two groups.
The clinical outcomes of patients stratified by in vitro antimicrobial susceptibility are presented in Table 2. Both the susceptible and non-susceptible groups had high clinical and microbiological success rates in response to the empirical antimicrobial treatments (overall clinical success rate=90.7%; overall microbiological success rate=97.7%). Fever subsided about 6 hours after the initiation of antimicrobials in both groups. The relapse rate was higher in the susceptible group than in the non-susceptible group, but a statistically significant difference was not observed. In total, 3 patients (7.0%) experienced relapse.
3. Patients who experienced relapse of urinary tract infection
Patients who experienced relapse after the completion of treatment with non-carbapenem treatment are presented in Table 3. One patient was resistant to definitive antimicrobial treatments (cefotaxime), whereas the other two patients were sensitive to definitive antimicrobial treatments (piperacillin/tazobactam) by in vitro antimicrobial susceptibility test results. None of these patients had an underlying urologic disorder, a history of UTIs, or previous hospitalization history. All of them had abnormal image findings on USG: two had thickened renal pelvis walls and one had hydronephrosis in the left kidney. Two patients underwent DMSA and VCUG, and the results were normal. All the relapse patients achieved clinical and microbiological success with cefotaxime as the empirical antimicrobial treatment. The two later switched to piperacillin/tazobactam based on the results of their in vitro antimicrobial susceptibility tests. When they relapsed, one patient who was resistant to the definitive treatment received piperacillin/ tazobactam and two patients who were sensitive to the definitive treatment were treated with ertapenem. All three patients were found to be sensitive to the treated antimicrobials by in vitro antimicrobial susceptibility test and no longer relapsed.
Discussion
Carbapenems are considered a gold standard treatment for infections due to ESBL-producing strains, even if the patient's infection is already clinically responsive to other β-lactams [25,26]. However, indiscriminate carbapenem use dramatically increases the prevalence of carbapenem-resistant Enterobacteriaceae, which poses a serious threat to public health. Clinicians should carefully consider alternative strategies to minimize carbapenem use [11-13,27,28]. Another problem with carbapenem use also rises from a practical perspective. Carbapenems are designed to be used only intravenously; no other option for treatment is available if the patient cannot be hospitalized immediately or if a peripheral or central intravenous catheter can not be placed. On the other hand, non-carbapenems can be administered orally as well as intravenously [29]. Therapeutic options using non-carbapenems for UTIs caused by ESBL-producers is of great importance for the pediatric population, which commonly suffers from peripheral or central intravenous catheterization failure in clinical practice due to smaller and less visible veins [30,31].
Few studies have been conducted on the efficacy of noncarbapenem treatment for UTIs caused by ESBL-producing organisms in children (Table 4). Our results are generally consistent with previous reports, confirming that even without carbapenem treatment, children with UTIs caused by ESBL-producers had high clinical and microbiological success rates and low relapse rates. Tratselas et al. reported that although most of the patients (24 of 28) in their study (children with UTI caused by ESBL-producers) received empirical antimicrobial treatments that were incongruent to their in vitro antimicrobial susceptibility test findings, the overall clinical and microbiological success rates were 94.4% and 95.0%, respectively. At follow-up, the formation of kidney scars in these patients did not significantly differ compared with children with UTIs caused by non-ESBL-producers [20]. Peco-Antić et al. suggested that the in vitro resistance of ESBL-producing E. coli to ceftriaxone, a thirdgeneration cephalosporin, did not necessarily indicate in vivo sensitivity. They found that clinical and microbiological responses to ceftriaxone were similar between UTIs caused by ESBL-producers and those by non-ESBL-producers [17]. These results are also consistent with previous studies in South Korean populations. Lee et al. and Han et al. confirmed that children treated with non-carbapenems for UTIs caused by ESBL-producing strains showed favorable clinical outcomes [21,22]. Hyun et al. reported that the relapse rate in children with UTIs caused by ESBL-producers did not differ significantly with those treated with susceptible antimicrobials or with those treated with nonsusceptible but clinically effective antimicrobials [23]. These findings are in line with those of studies on the use of cephalosporin or β-lactam/ β-lactamase inhibitors for the treatment of ESBL infections in adults, with the conclusion that several non-carbapenems are not inferior to carbapenems and deserve consideration as an alternative options for ESBL infections, depending on the source of infection and severity of illness [32-35].
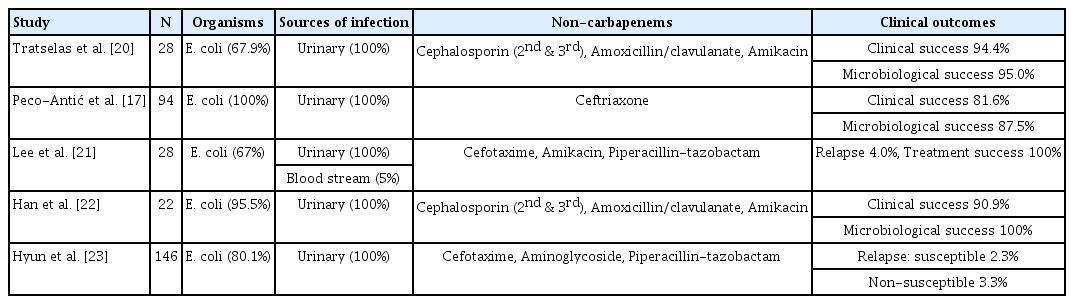
Previous studies evaluating the efficacy of non-carbapenem treatment for urinary tract infections caused by extended-spectrum β-lactamase producing Enterobacteriaceae
In this study, the overall relapse rate was 7.0% (3 of 43). We found no significant differences in the clinical characteristics and treatment outcomes according to the results of in vitro antimicrobial susceptibility test except for Creactive protein level between the susceptible and nonsusceptible groups. Both groups had high clinical and microbiological success rates regardless of the results of in vitro antimicrobial susceptibility test (overall 90.7% and 97.7%, respectively). Our findings suggest that non-carbapenems may be useful as alternative therapy for UTIs caused by ESBL-producing E. coli , if clinical improvements are presented.
Although the data from our study will add value to efforts to spare carbapenem as few studies have studied the efficacy of non-carbapenem treatments with UTIs caused by ESBL-producers in pediatric population, this study has several limitations. First, this is a retrospective observational study from a single center with a small sample size. Second, due to very small number of relapsed patients, risk factors for relapse after non-carbapenem treatment could not be evaluated. Although most patients were followed up for a month after discharge, there is a possibility that some patient visited another hospital when there were symptoms of relapse. Third, because identification for the type of β- lactamase enzymes in E. coli was not performed, their association with clinical outcomes cannot be assessed.
In conclusion, we found that non-carbapenem treatment for UTIs caused by ESBL producing E. coli in children under 2 years of age had high clinical and microbiological success rates, regardless of in vitro antimicrobial susceptibility test results, with an overall relapse rate of 7.0%. These results provide evidence that non-carbapenems are viable alternative treatments for UTIs caused by ESBL-producing E. coli . However, further prospective studies with large sample sizes and in-depth investigation of laboratory evidence are required to establish conclusive findings.
Notes
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-forprofit sectors.
Conflict of interest
No potential conflict of interest relevant to this article was reported.