| Child Kidney Dis > Volume 28(1); 2024 > Article |
|
Abstract
Purpose
The ability to concentrate urine becomes an important index in determining nocturnal enuresis (NE) treatment. The aim of our study was to investigate first-morning urine osmolality (Uosm) changes at the end of treatment compared to before treatment in children with NE.
Methods
A total of 71 children with NE were divided into two groups according to the level of first-morning Uosm before treatment: high group (Ōēź800 mOsm/kg) and low group (<800 mOsm/kg). Baseline parameters were obtained from uroflowmetry, frequency volume charts for at least 2 days, and a questionnaire for lower urinary tract symptoms. All patients were basically treated with standard urotherapy and medication. The first-morning Uosm was measured twice, before treatment and at the end of treatment.
Results
The response rate was higher in the low group after 3 months of treatment than in the high group (P=0.041). However, there was no difference between the two groups at the end of the treatment. In the high group, the first-morning Uosm at the end of treatment did not show a significant change compared to before treatment. In contrast, the first-morning Uosm increased in the low group at the end of treatment (P<0.001). However, it was still lower than that of the high group (P=0.007).
Nocturnal enuresis (NE) is identified as intermittent incontinence that occurs exclusively during sleeping periods [1]. The prevalence of NE has been reported to be about 5.6% among children aged 5 to 13 years [2]. Although NE is neither fatal nor life-threatening, it does present a significant risk of psychosocial depression in patients and families, and thus, immediate, adequate treatment is required [3].
The three main mechanisms in the pathophysiology of enuresis are excessive nocturnal urine production, low bladder capacity or increased detrusor activity, and arousal impairment [4]. Recent studies found much higher urine volumes and much lower osmolality values in children with NE compared to the normal population. The findings were related to the disruption of vasopressin [5,6]. The children with NE with preserved bladder storage function have reduced urine-concentrating ability during the night due to a lack of an arginine vasopressin (AVP) effect [5].
Urine osmolality (Uosm) provides a measure of the number of dissolved molecules in urine per unit of water and urine concentration. Uosm is more accurate than specific gravity and can be used to diagnose a variety of urinary concentration-associated disorders [7]. In several studies, Uosm was used instead of plasma AVP concentrations because Uosm can provide information on AVP [8-10]. Due to methodological difficulties that make urine collection from enuresis episodes a particularly demanding task, nocturnal urine output has previously been approximated through first-morning urine in patients with NE [11]. In addition, measuring Uosm is a simple, non-invasive, routine, and low-cost test that may help guide the optimal treatment of NE [12].
Several previous studies reported a relationship between nocturnal urinary concentrating ability and NE [5,13,14], but no study has investigated how much urinary concentrating ability, especially first-morning Uosm, improves after treating NE patients with low nocturnal urinary concentrating ability. In our previous study, we found that a significantly higher percentage of NE patients who have low first-morning Uosm had a response rate of Ōēź50% at 1 month and 3 months [6]. With an interesting result, we investigated further the relationship between first-morning Uosm and NE. Finally, the aim of our study was to investigate the response rate of NE treatment according to first-morning Uosm before treatment and first-morning Uosm changes at the end of treatment compared to before treatment in children with NE.
After obtaining approval from the Institutional Review Board of Pusan National University Yangsan Hospital (IRB No. 05-2023-032), a retrospective chart review was performed on the prospective cohort data of all children who underwent treatment for NE at our institution from September 2019 to May 2022. Seventy-one children with NE (>3 times/wk) with measurements of the first-morning Uosm before treatment and at the end of treatment were included in this study. Patients diagnosed with organic causes, such as congenital urinary tract anomalies, congenital or acquired neurologic disorders, urinary tract infections, or spinal bifida occulta, were excluded.
All patients completed a questionnaire and a 48-hour frequency/volume (48-h F/V) chart. The questionnaire included items on medical history and urinary symptoms, including frequency, daytime incontinence, urgency, urge incontinence, holding maneuver, and dysfunctional voiding scoring system (DVSS) score. The questionnaire responses and 48-h F/V chart findings were used to confirm the presence of lower urinary tract symptoms (LUTS). Constipation was evaluated using the Leech scores of abdominal X-ray findings for all patients [15].
First-morning urine samples were collected twice from all patients, on the second hospital visit and the final hospital visit at the end of treatment. Patients and parents were instructed to collect first-morning samples in the plastic cups provided and to keep them refrigerated at 4 Ōäā. Samples were evaluated promptly upon arrival at the hospital.
The 71 patients were divided into two groups according to first-morning Uosm values before treatment: (1) the high group, with a first-morning Uosm of Ōēź800 mOsm/kg, and (2) the low group, with a first-morning Uosm of <800 mOsm/kg before treatment. Our previous study, which divided the groups based on 800 mOsm, showed significant results, so we divided the groups as before [6]. Daytime maximum voided volume (VV), first-morning VV, and total urine volume were obtained from 48-h F/V charts. Uroflowmetry (UFM) and post-void residual volume (PVR) findings, maximum flow rates (Qmax), VV, average flow rate (Qave), and PVRs were also analyzed.
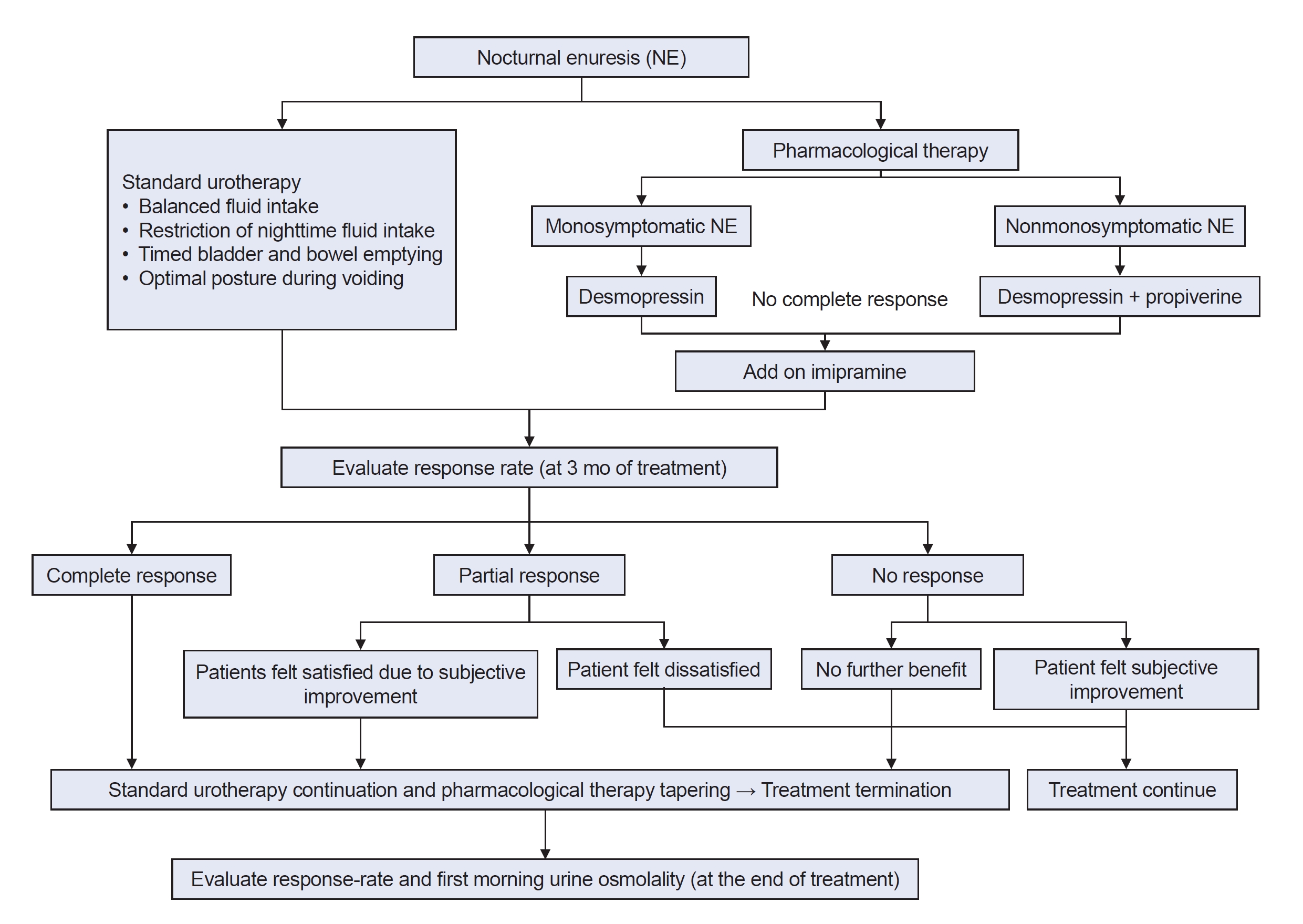
Following the before-treatment evaluations of patient characteristics, 48-h F/V charts, UFM and PVR, standard urotherapy, and pharmacological therapy were provided in accordance with International ChildrenŌĆÖs Continence Society (ICCS) recommendations. Standard urotherapy included an introduction to LUTS treatment and lifestyle modifications (balanced fluid intake, restriction of nighttime fluid intake, timed bladder and bowel emptying, and optimal posture during voiding). In this study, the alarm treatment, also known as first-line treatment, was applied in cases that did not respond to pharmacological therapy.
Primary pharmacological therapy included desmopressin (1-desamino-8-D-arginine vasopressin, dDAVP), propiverine, and/or imipramine. All patients were treated with desmopressin 120 ╬╝g at first. The desmopressin dose was increased or decreased (60 ╬╝g or 240 ╬╝g) depending on the patient's response to the agent. If there was no response (NR) to desmopressin, propiverine or imipramine was added short-term as needed. These drugs were used based on consideration of symptom severity, the presence of any other LUTS, a history of bladder dysfunction.
The response rates were assessed at 3 months and at the end of treatment. The response rate was calculated as a percentage of the reduced rate of current enuresis events compared to the initial enuresis event [response rate=100├Ś(number of initial enuresis event per weekŌĆōnumber of current enuresis event per week)/number of initial enuresis event per week]. The patients were categorized into three groups according to ICCS recommendations: complete response (CR), partial response (PR), or NR groups. CR was defined as a 100% reduction in enuresis. PR was defined as a 50% to 99% reduction in enuresis, and NR was defined as a <50% reduction in enuresis (Fig. 1).
Pharmacological therapy was terminated when the child showed consistent findings of CR. In PR, treatment was terminated when the patients felt satisfied. In NR, treatment was continued as long as the patient reported subjective improvement. And treatment was terminated when there was no further benefit. At the end of treatment, the patients were maintained on urotherapy alone without pharmacological therapy. The first-morning Uosm at the last visit was re-examined and compared with the first-morning Uosm before treatment (Fig. 1).
SPSS version 27 (IBM Corp.) was used for the statistical analyses. P-values of <0.05 were considered statistically significant. Continuous variables were analyzed using the Student t-test and the Mann-Whitney U test. Categorical variables were analyzed using the Pearson chi-square test and Fisher exact test.
There were no differences in patient characteristics, including age, height, weight, body mass index, follow-up period, constipation, and enuresis frequency, at the first visit between the two groups except for sex (P=0.025) (Table 1). There were also no differences in LUTS, such as urine frequency, urgency, daytime incontinence, urge incontinence, holding maneuver, and DVSS scores, between the two groups (Table 1). In the 48-h F/V chart, the first-morning VV and total urine volume in the low group were significantly higher than in the high group (P=0.049 and P=0.024, respectively). In the UFM test, there was no difference between the two groups in Qmax, VV, delay time, flow time, voiding time, and flow index, except for Qave (P=0.015). There was also no difference between the two groups in PVR (Table 2).
Enuresis frequency improved with treatment in both groups. Enuresis frequency in the low group was relatively lower at 3 months of treatment than in the high group (0.73┬▒1.10 times/wk vs. 1.33┬▒1.47 times/wk, P=0.027) (Fig. 2). However, these enuresis frequencies were not different between the groups at the end of treatment (0.89┬▒1.93 times/wk vs. 0.49┬▒0.75 times/wk, P=0.815) (Fig. 2). In the low group, enuresis frequency was not different between 3 months and the end of treatment (Fig. 2). The response rate was higher in the low group at 3 months of treatment than in the high group (82.2%┬▒22.0% vs. 66.0%┬▒34.0%, P=0.041) (Table 3). However, there was no difference between the two groups at the end of the treatment. Moreover, at the end of treatment, there was no significant difference between the two groups in the number of patients with CR, PR, and NR (Table 3).
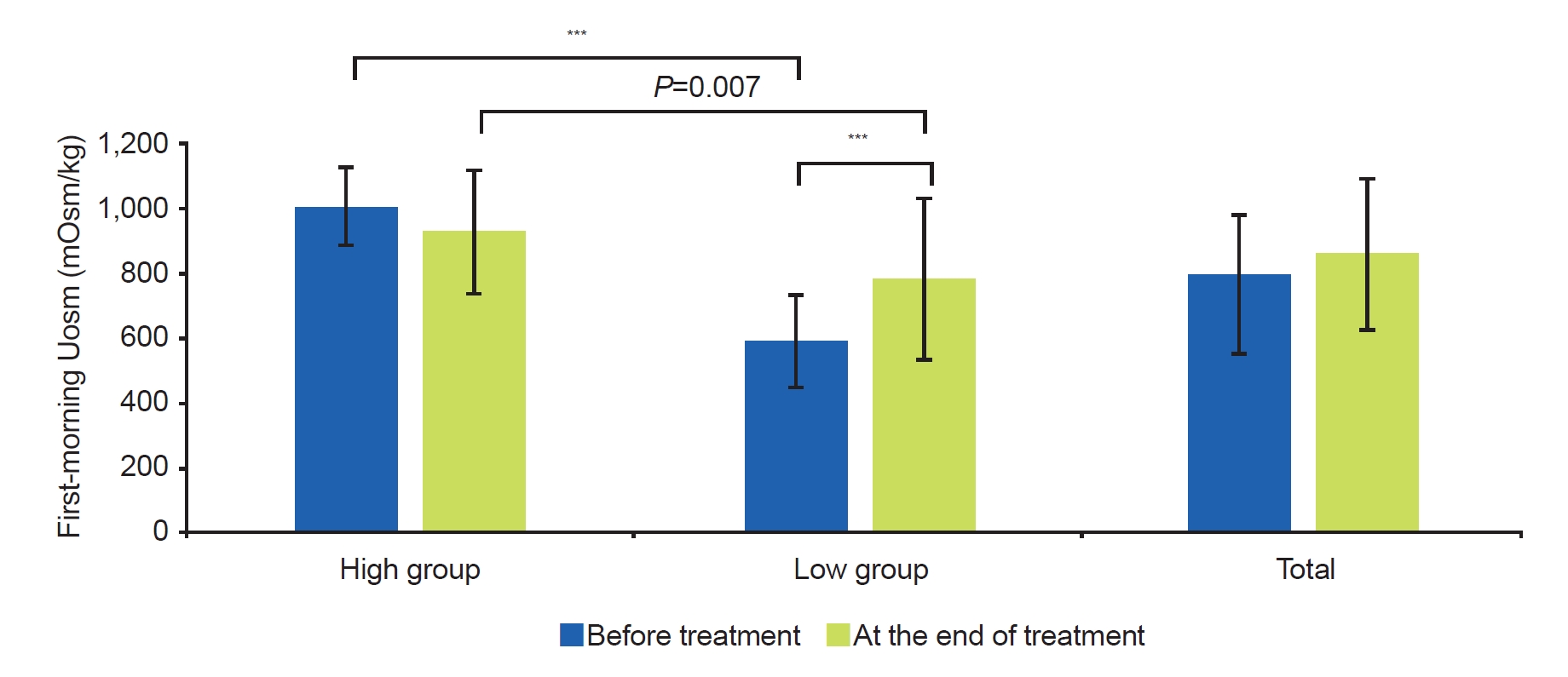
In the high group, the first-morning Uosm at the end of treatment did not show a significant change compared to before treatment. In contrast, the first-morning Uosm at the end of treatment was increased in the low group compared to before treatment (586.8┬▒147.2 mOsm/kg vs. 780.2┬▒249.1 mOsm/kg, P<0.001). However, the first-morning Uosm at the end of treatment in the low group was lower than in the high group (780.2┬▒249.1 vs. 926.9┬▒190.4, P=0.007) (Fig. 3). Therefore, Uosm in the low group did not reach the Uosm level in the high group at the end of NE treatment.
In our study, Uosm increased after treatment. The first-morning Uosm in the low group increased at the end of treatment (586.8┬▒147.2 mOsm/kg vs. 780.2┬▒249.1 mOsm/kg, P<0.001). This study was the first to investigate changes by measuring first-morning Uosm before treatment and at the end of treatment. In our study, the response rate of the low group was better at 3 months of treatment than that of the high group. Also, enuresis frequency in the low group improved more at 3 months of treatment than in the high group. At the end of treatment, the first-morning Uosm increased in the low group. This means that improvements in excessive nocturnal urine production, one of the causes of NE, can be predicted through first-morning Uosm before treatment. Predicting and evaluating treatment response at 3 months of treatment is important because treatment adherence is better when short-term treatment effects are good.
It is well known that a low ability to concentrate nocturnal urine is one of the main mechanisms of NE. Several studies have investigated Uosm as a predictor of NE treatment, but the results have been contradictory [7,8]. Dehoorne et al. [13] described 42 children with monosymptomatic NE (MNE) and night polyuria with high Uosm (>850 mmol/L) not responding to intranasal dDAVP. Thus, nocturnal polyuria with high urinary osmolality with desmopressin-resistant MNE is related to abnormally increased osmotic excretion. In a study of 67 children with enuresis, Sozubir et al. [14] reported a significantly higher number of responders to dDAVP treatment when the Uosm value was <800 mOsm/kg. In their study, lower spot Uosm was the only statistically significant predictor of the desmopressin response. A study by Neveus et al. [5] that included 12 children with enuresis reported a significantly lower baseline Uosm (553┬▒134 mOsm/kg) in dDAVP responders compared to non-responders (920┬▒226 mOsm/kg).
However, Unuvar and Sonmez [16] in a study of 55 NE children and 15 healthy children between the ages of 5 and 15 years investigating Uosm in both daytime and nighttime urine, reported that pretreatment urine volume osmolality values were not predictive factors of response to desmopressin or conditioning therapy. A study of 35 children with enuresis by Eller et al. [8] reported that 27 children demonstrated a CR to desmopressin treatment at doses of 10ŌĆō30 ╬╝g. However, spot Uosm values were not predictive of the desmopressin response. Urine samples were collected at home at times that would best reflect fluctuations in plasma vasopressin levels (8:00, 16:00, and 22:00) [8]. In a study by Folwell et al. in 31 NE patients [17], the mean and peak Uosm of the morning urine samples showed no difference while on treatment with dDAVP compared to placebo. They suggested that early morning Uosm, as a reflection of changes in nocturnal osmolality, was not useful in selecting patients who would respond to treatment. Medel et al. [18] investigated seven healthy children, six primary NE children who were desmopressin responders, and five primary NE children who were desmopressin non-responders. They found no significant difference in mean Uosm at night (from midnight to 8:00 AM). Therefore, they suggested that baseline Uosm was not a significant predictor of response to dDAVP therapy.
In our study, baseline Uosm was a significant predictor of response to treatment. Especially at 3 months of treatment, children with low Uosm showed low enuresis frequency (1.33┬▒1.47 times/wk vs. 0.73┬▒1.10 times/wk, P=0.027) and a high response rate (66.0%┬▒34.0% vs. 82.2%┬▒22.0%, P=0.041). However, there was no difference at the end of the treatment. The interesting result was that the Uosm range was very wide for each person. In our study, the childrenŌĆÖs first-morning Uosm range was 160ŌĆō1,261 mOsm/kg (mean, 797.7 mOsm/kg). Despite treatment with dDAVP, not all children showed an increase in Uosm, and some children had decreased Uosm at the end of treatment. Several reasons can be considered as the causes of this variation. First, the first-morning urine in our study did not reflect all urine that occurred at night. Unlike other studies that collected all urine from midnight to 8:00 AM, our study did not collect all urine that occurred at night. Since night leaks were not collected, the time at which the first-morning urine was produced may have varied from child to child. Second, the collection time of the first-morning urine also varied from child to child. The time to collect the first-morning urine could be different, depending on the waking time of the child. These issues may have caused unexpected biases. Nevertheless, there is a lot of information available in first-morning urine, and the advantages of a simple, non-invasive, and low-cost test are clear. Therefore, it is meaningful to predict the treatment outcome of NE through the first-morning urine in clinical practice.
The guidelines published by the ICCS in 2011 recommend the use of an enuresis alarm or desmopressin, a vasopressin analog, as the standard treatment for MNE and are particularly recommended for patients with nocturnal polyuria [19]. Desmopressin is an efficient and safe treatment for primary MNE, with a reported success rate of 70% to 75% [16]. One of the major actions of desmopressin is to reduce the volume of urine produced overnight to within normal limits [20]. Desmopressin acts on the V2 receptors of the distal tubules and collecting tubules of the kidney, leading to urine concentration and decreased urine volume through the water channel aquaporin 2 [21]. Moreover, desmopressin positively influences the abundance of key sodium transporters in the thick ascending limb and collecting duct. Such an effect appears reasonable as part of the mechanism responsible for the buildup of the medullary osmotic gradient, the driving force for water reabsorption. [22].
Few studies have observed changes in Uosm after treatment with dDAVP. Kamperis et al. [22] reported that Uosm increased significantly at night only after the administration of dDAVP in a group with nocturnal polyuria (from 559┬▒70 mOsm/kg to 876┬▒39 mOsm/kg, P<0.001), whereas no significant changes were observed in the controls. This study showed changes in Uosm during one night. In one Korean study, Uosm in the dDAVP-complete responder group was lower than that of the non-responder group before treatment (461.2┬▒192.7 mmol/L vs. 773.5┬▒235.8 mmol/L). Moreover, 2 weeks after starting treatment, Uosm in the dDAVP-complete responder group was significantly increased (from 461.2┬▒192.7 to 591.2┬▒159.8 mmol/L) [23]. Since these two studies investigated Uosm during treatment, there is a limitation that the changes in Uosm at the end of treatment are not known.
A limitation of this study is that it was a single-center, retrospective study, and the number of patients was limited. In the future, a large-scale prospective study should be performed.
In conclusion, the ability to concentrate nocturnal urine improved at the end of treatment compared to before treatment in the low Uosm NE children. NE improved faster in the low Uosm group before treatment than in the high group. However, there was no difference in the treatment effect between the two groups at the end of treatment. In the low group, first-morning Uosm increased after treatment, but it did not reach the level in the high group.
Notes
Ethical statements
After obtaining approval from the Institutional Review Board of Pusan National University Yangsan Hospital (IRB No. 05-2023-032), a retrospective chart review was performed on the prospective cohort data of all children who underwent treatment for nocturnal enuresis at our institution.
Funding
This study was supported by a 2023 research grant from Pusan National University Yangsan Hospital.
Author contributions
Conceptualization: JMC
Data curation: YHL, JMC
Formal analysis: YHL
Funding acquisition: JMC
Investigation: YHL, JMC
Methodology: JMC
Project administration: JMC
Visualization: YHL
Writing-original draft: YHL
Writing-review & editing: JMC, SDL
All authors read and approved the final manuscript.
References
1. Austin PF, Bauer SB, Bower W, Chase J, Franco I, Hoebeke P, et al. The standardization of terminology of lower urinary tract function in children and adolescents: update report from the standardization committee of the International ChildrenŌĆÖs Continence Society. Neurourol Urodyn 2016;35:471-81.


2. Chung JM, Lee SD, Kang DI, Kwon DD, Kim KS, Kim SY, et al. An epidemiologic study of voiding and bowel habits in Korean children: a nationwide multicenter study. Urology 2010;76:215-9.


3. Van Herzeele C, Walle JV, Dhondt K, Juul KV. Recent advances in managing and understanding enuresis. F1000Res 2017;6:1881.



4. Arda E, Cakiroglu B, Thomas DT. Primary nocturnal enuresis: a review. Nephrourol Mon 2016;8:e35809.



5. Neveus T, Lackgren G, Tuvemo T, Stenberg A. Osmoregulation and desmopressin pharmacokinetics in enuretic children. Pediatrics 1999;103:65-70.


6. Lee GK, Chung JM, Lee SD. First-morning urine osmolality and nocturnal enuresis in children: a single-center prospective cohort study. Investig Clin Urol 2023;64:501-9.



7. Pagana KD, Pagana TJ. Mosby's manual of diagnostic and laboratory tests. 7th ed. Elsevier Health Sciences; 2017.
8. Eller DA, Homsy YL, Austin PF, Tanguay S, Cantor A. Spot urine osmolality, age and bladder capacity as predictors of response to desmopressin in nocturnal enuresis. Scand J Urol Nephrol Suppl 1997;183:41-5.

9. Kawauchi A, Watanabe H, Miyoshi K. Early morning urine osmolality in nonenuretic and enuretic children. Pediatr Nephrol 1996;10:696-8.


10. Steffens J, Netzer M, Isenberg E, Alloussi S, Ziegler M. Vasopressin deficiency in primary nocturnal enuresis: results of a controlled prospective study. Eur Urol 1993;24:366-70.


11. Eggert P, Kuhn B. Antidiuretic hormone regulation in patients with primary nocturnal enuresis. Arch Dis Child 1995;73:508-11.



12. Abdovic S, Cuk M, Hizar I, Milosevic M, Jerkovic A, Saraga M. Pretreatment morning urine osmolality and oral desmopressin lyophilisate treatment outcome in patients with primary monosymptomatic enuresis. Int Urol Nephrol 2021;53:1529-34.


13. Dehoorne JL, Raes AM, van Laecke E, Hoebeke P, Vande Walle JG. Desmopressin resistant nocturnal polyuria secondary to increased nocturnal osmotic excretion. J Urol 2006;176:749-53.


14. Sozubir S, Ergun G, Celik A, Ulman I, Avanoglu A. The influence of urine osmolality and other easily detected parameters on the response to desmopressin in the management of monosymptomatic nocturnal enuresis in children. Minerva Urol Nefrol 2006;58:207-12.

15. Leech SC, McHugh K, Sullivan PB. Evaluation of a method of assessing faecal loading on plain abdominal radiographs in children. Pediatr Radiol 1999;29:255-8.


16. Unuvar T, Sonmez F. The role of urine osmolality and ions in the pathogenesis of primary enuresis nocturna and in the prediction of responses to desmopressin and conditioning therapies. Int Urol Nephrol 2005;37:751-7.


17. Folwell AJ, Macdiarmid SA, Crowder HJ, Lord AD, Arnold EP. Desmopressin for nocturnal enuresis: urinary osmolality and response. Br J Urol 1997;80:480-4.


18. Medel R, Dieguez S, Brindo M, Ayuso S, Canepa C, Ruarte A, et al. Monosymptomatic primary enuresis: differences between patients responding or not responding to oral desmopressin. Br J Urol 1998;81 Suppl 3:46-9.


19. Neveus T, Eggert P, Evans J, Macedo A, Rittig S, Tekgul S, et al. Evaluation of and treatment for monosymptomatic enuresis: a standardization document from the International ChildrenŌĆÖs Continence Society. J Urol 2010;183:441-7.


20. Vande Walle J, Rittig S, Bauer S, Eggert P, Marschall-Kehrel D, Tekgul S, et al. Practical consensus guidelines for the management of enuresis. Eur J Pediatr 2012;171:971-83.



21. Akagawa S, Tsuji S, Akagawa Y, Yamanouchi S, Kimata T, Kaneko K. Desmopressin response in nocturnal enuresis showing concentrated urine. Pediatr Int 2020;62:701-4.


Fig.┬Ā2.
Improvement of enuresis frequency at 3 months of treatment and at the end of treatment. *P<0.05.
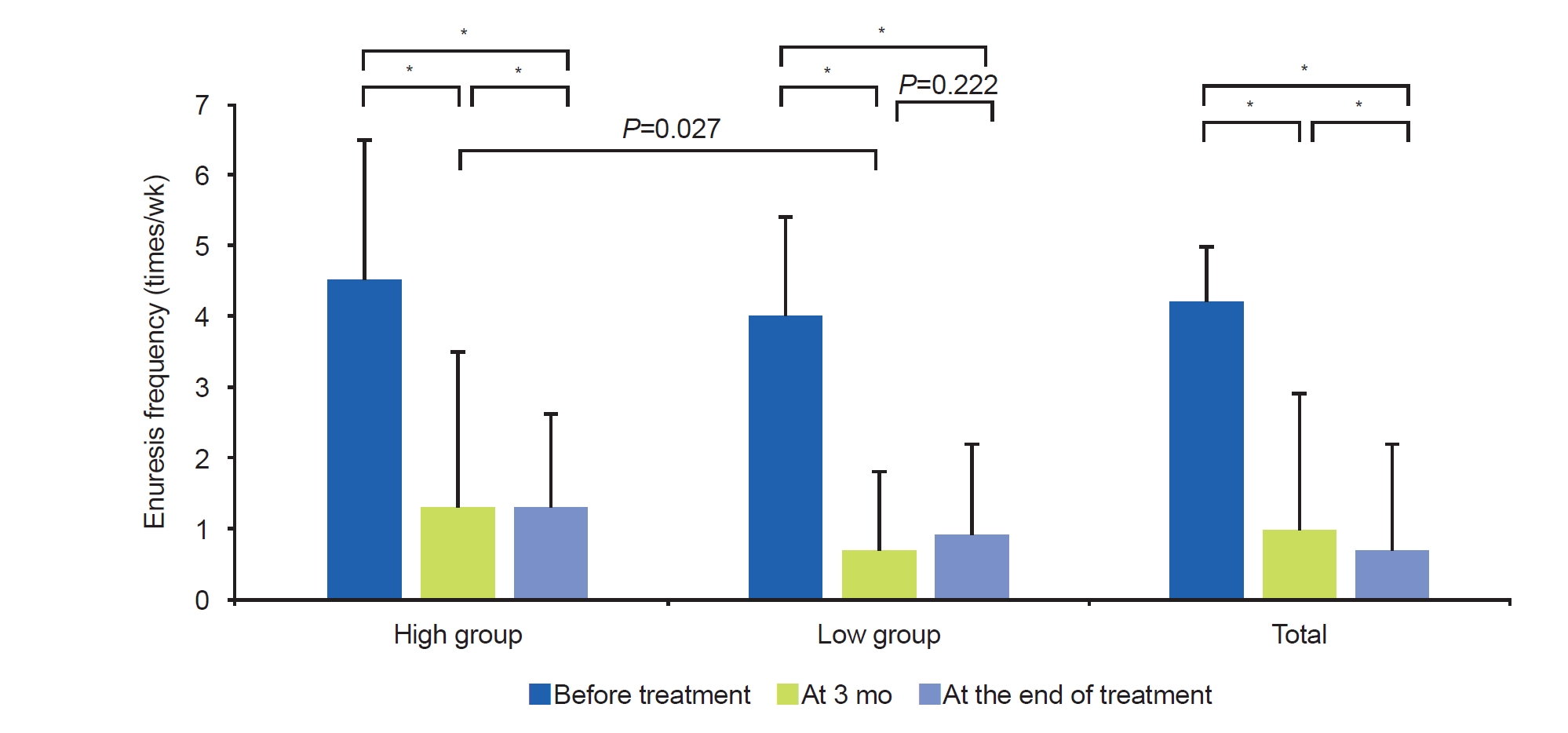
Fig.┬Ā3.
Change of first-morning urine osmolality (Uosm) before treatment and at the end of treatment. ***P<0.001.
Table┬Ā1.
Patients characteristics at first visit
Table┬Ā2.
Frequency volume chart and uroflowmetry
Table┬Ā3.
Treatment outcome
- TOOLS
-
 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link XML Download
XML Download Full text via DOI
Full text via DOI-
 Download Citation
Download Citation
- Download Citation
-
- Close
 Print
Print-
Share :
-
METRICS

-
- 0 Crossref
- 0 Scopus
- 952 View
- 9 Download
- ORCID iDs
-
Yun ha Lee

https://orcid.org/0000-0001-8709-7009Jae Min Chung

https://orcid.org/0000-0002-7467-5954Sang Don Lee

https://orcid.org/0000-0001-9459-3887 - Related articles