Fecal Retention in Overactive Bladder (OAB) in Children: Perspective of a Pediatric Gastroenterologist
Article information
Abstract
Coexisting voiding and bowel dysfunction in children are common in the clinic. The idea that overactive bladder (OAB) and constipation arise from one single pathophysiology has been reinforced in many studies. In Korea, a nationwide multicenter study conducted in 2009 showed that overall prevalence of OAB in children, 5-13 years of age, was 16.59% and this number has increased more recently. The initial step to manage coexisting fecal retention and OAB in children is to characterize their bowel and bladder habits and to treat constipation if present. Although diagnosing constipation in children is difficult, careful history-taking using the Bristol Stool Form Scale, and a scoring system of plain abdominal radiography, can help to estimate fecal retention more easily and promptly. Non-pharmacological approaches to manage functional constipation include increasing fluids, fiber intake, and physical activity. Several osmotic laxatives are also effective in improving OAB symptoms and fecal retention. Additionally, correction and education in relation to toilet training is the most important measure in treating OAB with fecal retention.
Introduction
Many studies have reported a close association between voiding and functional gastrointestinal disorders [1-3]. Especially, voiding and bowel dysfunction in children have increased recently, possibly due to lifestyle changes including feeding patterns and environmental factors. Both conditions can significantly impact on health-related quality of life (HRQL), and their coexistence may further aggravate adverse outcomes in affected children and their family [4-6].
In 2002, the International Continence Society reported a consensus definition for the symptoms associated with overactive bladder (OAB), and this included urinary urgency, with or without urge incontinence (UI), usually with urinary frequency and nocturia, in the absence of pathologic or metabolic factors [7]. Large epidemiological studies of OAB in adults have reported a prevalence of 9-16% [8]. But studies investigating prevalence rates of OAB in schoolchildren and adolescents are scarce. In fact, the assessment of OAB in a child is more difficult than in an adult. The initial signs and symptoms of OAB in children can vary dramatically, and, therefore, are difficult to define objectively. In Korea, a nationwide multicenter study carried out in 2009 showed that overall prevalence of OAB in children, 5-13 years of age, was 16.59% and decreased with age [9].
Constipation is also a common problem in children worldwide, and its prevalence in the general population has been reported to range from 0.7% to 29.6% [10]. However, this prevalence varies widely, partly because of differences in survey methods, population, age, and the definitions used. The diagnosis of constipation is difficult because it depends on the simple description by parents or guardians and on various defecation symptoms reported by the children themselves. Although, constipation can be diagnosed by various methods, it remains difficult to diagnose in clinics.
It is well known that OAB is closely associated with constipation [1-3]. Previous studies have shown that voiding symptoms are improved only by treating constipation [3]. Constipation induces bladder dysfunction by the following mechanism: when the rectum is filled with stools, stretch receptors are stimulated and transmitted to the brain, which induce temporary involuntary contraction of the external anal sphincter and the puborectalis muscles [11]. If defecation is not possible, the muscles contract voluntarily. As this process is repeated, the feces accumulate within the rectum and this shortens the bladder contraction period and ultimately bladder activity is stopped. Another hypothesis suggests that when the rectum is distended, it compress the adjacent bladder, and decreases the functional bladder capacity. If stretch receptors are stimulated by fecal accumulation, diverse patterns of bladder contraction occur and induce bladder instability [12].
These two mechanisms of OAB are mainly related to rectal distention caused by fecal retention. In clinical practices, many patients or their caregivers tend to disagree with the presence of constipation, when they are asked “Do you (your child) have constipation?”, even though they are concerned with irregular or long duration of defecation. In follow-up questions about the cohesion or form of stools or pain on defecation, almost all provide an answer suggestive of constipation or fecal retention. Therefore, it is reasonable that fecal retention not constipation is considered as the main factor in development of OAB. Some pediatric gastroenterologists used the term ‘occult constipation’ to characterize a state of fecal retention [13]. This was defined as no complaints of constipation at the initial medical history and no symptoms indicating presence of constipation, with at least one of the following signs: a) hard consistency of stools (described as "rock-like" or "pellet-like") on rectal examination, and b) plain abdominal radiograph showing a distended large intestine loaded with fecal material. For adequate treatment of OAB, it is necessary that an exact and objective tool is used to assess fecal retention, even though evaluating fecal loading during childhood may be difficult due to an unreliable history or embarrassment on the child’s part.
This review briefly describes the role of bladder-bowel interactions in clinical conditions, with an emphasis on fecal retention and OAB; and discusses the tools for diagnosing fecal retention. This information will be useful in the management of OAB in children with concomitant fecal retention.
1. Relationship between bladder and bowel function
The bladder and bowel both originated from the embryologic hindgut [14], and their function is closely related given the interaction of the neural network responsible for their normal functioning [15-17]. Both bladder function and intestinal function are controlled by the supraspinal regions, such as the anterior cingulate gyrus, prefrontal cortex, and the insular region of the cerebral cortex. Conscious control of both bladder and bowel function results from transmission of afferent impulses carrying sensory information to the brain [18-21].
Surprisingly, different subtypes of interstitial cells (known as interstitial cells of Cajal (ICC) or myofibroblasts) have been identified in the urinary bladder wall, including the lamina propria and detrusor muscle [22-24]. ICCs are known to play an important role in lower gastrointestinal tract function, acting as pacemakers that initiate slow-wave contractile activity in adjacent smooth muscle cells and act as mediators of signal transduction from neurons to smooth muscle cells. Studies of the function of ICCs in the urinary bladder of guinea pigs indicate that they demonstrate spontaneous electrical activity and Ca2+-signaling and respond to neurotransmitters [25, 26]. Although, the distinct patterns of spontaneous contractile activity of the urinary bladder are different from the slow-wave activity of the gastrointestinal tract, ICCs appear to act as mediators of normal contractile function in both organs [27]. Consequently, the dysfunction of both the bladder and the intestines may be a result of the same pathophysiology.
2. Association of fecal retention and OAB
It is generally accepted that fecal retention causes distension of the rectum, and, hence, bladder compression, furthermore, it is possible that this compression stimulates bladder stretch receptors, decreasing functional bladder capacity and, possibly, triggering detrusor contractions. Additionally, it is generally accepted that dysfunctional voiding results from overtraining the pelvic floor as a defense against urine loss. The resulting high pelvic floor muscle tone may cause dysfunctional elimination of feces, which can lead to chronic constipation, with or without encopresis [15]. Possible explanations for the association between constipation and OAB are summarized below. The notion that OAB and constipation arise from one single pathophysiology is reinforced by the successful use of electrotherapy in the treatment of both constipation and OAB [18]. In some cases it has been shown that the filled rectum may aggravate bladder function. A study where a balloon was inflated in the patient’s rectum, to simulate rectal fullness, showed that acute rectal distension affects bladder function in children, independent of the presence of chronic constipation, through an excitatory response to rectal distention [28]. Moreover, in patients with chronic constipation the rectum is never empty, and continuous anal sphincter contraction may occur, through the normal reflex mechanism and through voluntary action, and these can induce concomitant continuous contraction of the urethral sphincter.
Similarly, OAB may cause constipation. It is known that children with OAB contract the pelvic floor when performing holding movements to prevent urinary incontinence. Contraction of the anal sphincter muscles causes negative feedback, inhibiting rectal contraction and thus stimulating fecal retention [29]. Antimuscarinics, prescribed to treat OAB, are also known to cause constipation. Therefore, even if a patient with OAB does not experience constipation initially, the risk of developing constipation may increase with pharmacologic treatment of the OAB symptoms. Since colonic transit is mediated in part by cholinergic nerve activity in the bowel, medication to inhibit this activity may prolong colonic transit time and aggravate constipation [30]. The higher incidence of constipation observed with medication may relate to the greater affinity for the M3 receptor than for the other muscarinic receptor subtypes [31]. Another factor that contributes to the association between OAB and constipation is low fluid intake. Many children with OAB may feel anxious about drinking fluids during the day so as not to experience urinary incontinence, especially during school hours. The low fluid intake may in turn cause fecal retention or worsen constipation [32]. It is likely that the association between OAB and constipation is multifactorial.
3. Diagnostic approach to fecal retention in clinical practice
Several objective methods have been used to measure fecal loading in the clinic. These include rectal examination after defecation, rectal manometry with balloon insufflation and anal sphincter electromyography. However, these procedures can be distressing to the child. Comprehensive history should include questions related to the frequency and characteristics of stools. Methods based on form and density of the stools, such as the Bristol Stool Form Scale, can be helpful to estimate fecal retention more easily and promptly, but rely on the description by the patient or an observer, and could be confounded by a temporary change of defecation habits. Abdominal examination is useful to assess abdominal distention caused by gaseous or fecal impaction. Constipation or fecal retention can be identified as a palpable fecal mass present on the left lower quadrant, around the sigmoid colon.
More objectively, plain abdominal radiography has been used to estimate the amount of fecal retention in the bowel [33]. There are several scoring systems for the quantification of the amount of feces in the colon, such as Barr, Blethyn and Leech scoring. Amongst the three systems, the Leech scoring system has been reported as reproducible, and with the highest sensitivity and specificity, for the diagnosis of functional constipation [34].
1) Scoring of fecal retention in plain abdominal radio graphy
The first method was described by Barr, and quantifies the feces amount and consistency (i.e. granular or rocky stools) in four different bowel segments. Constipation is defined as a Barr score of more than 10. The second method, described by Blethyn [34], is a rough scoring system used to assess the amount of feces in the large bowel. In this system, the degree of fecal retention is graded as follows: grade 0, normal (feces in the rectum and cecum only); grade 1, mild constipation (feces in the rectum and cecum, and discontinuous collection of feces elsewhere); grade 2, moderate constipation (feces in the rectum and cecum, continuous collection of feces in all segments); and grade 3, severe constipation (feces in the rectum and cecum, with continuous collection elsewhere, and a dilated colon and impacted rectum). The third scoring system was described by Leech [34], where the colon is divided into three colonic segments (ascending and proximal transverse colon, distal transverse and descending colon, and rectosigmoid colon). The amount of feces in each segment is then scored from 0 to 5: 0, indicating no feces; 1, scanty feces visible; 2, mild fecal loading; 3, moderate fecal loading; 4, severe fecal loading; and 5, severe fecal loading with bowel dilatation. The combined score range is from 0 to 15, where a score above 8 indicates constipation (Fig. 1).
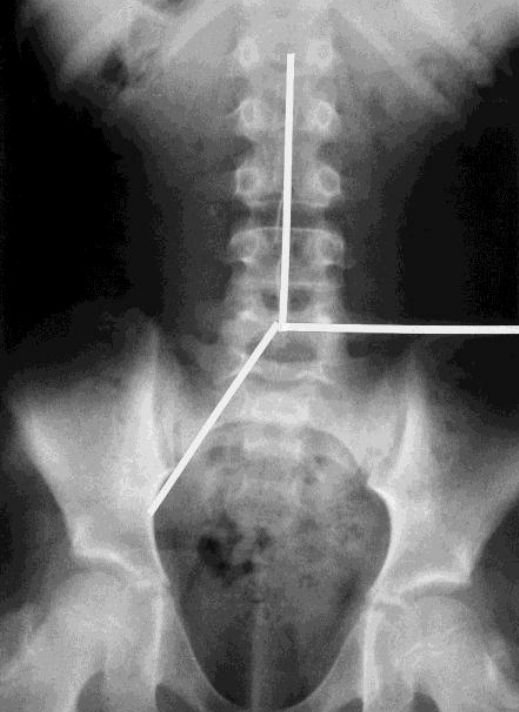
Plain abdominal radiograph with the division of the abdomen into three segments. A vertical line was drawn upwards from the body of the 5th lumbar vertebra, dividing the upper part of the abdomen into two. The area below the pelvic brim on the right and the anterior superior iliac spine on the left defined the rectosigmoid and rectum.
2) Bristol Stool Form Scale
Stool characteristics are defined on a 7-point scale, in which stools are scored according to consistency, form and surface smoothness, as follows: 1, separate hard lumps (like nuts); 2, sausage-shaped, but lumpy; 3, like a sausage or snake, but with cracks on its surface; 4, like a sausage or snake, and smooth and soft; 5, soft blobs with a clear cut edge; 6, fluffy pieces with ragged edges and mushy; and 7, watery with no solid pieces (Fig. 2) [35].
4. Implications for managing fecal retention and OAB
In children with combined bowel bladder dysfunction, after identifying current bowel and bladder habits, the initial treatment should address fecal retention or constipation [36]. The Rome III criteria for diagnosing functional constipation in children using a reliable questionnaire considers constipation present when the child presents at least two of the six symptoms for longer than two months (Table 1) [37]. However, many children with fecal retention do not meet this criteria and further steps should be taken to consider fecal retention through the Leech scoring system in plain abdominal radiography and/or the Bristol Stool Form Scale. The duration of treatment is usually at least 3 to 6 months with frequent relapses [38]. Non-pharmacological approaches to manage functional constipation include increasing fluids, fiber intake, and physical activity [39]. Increasing fluids can be a concern in patients with OAB symptoms, who may feel anxious about drinking water, and this can exacerbate constipation. It is controversial whether the age of toilet training is associated with dysfunctional voiding in children. Hodges et al. suggested that starting toilet training prior to 24 months or later than 36 months of age was associated with dysfunctional voiding [40]. Even though, the children gained the toilet training at the best time, children who have not been trained correctly present with enuresis, urinary infection, voiding dysfunction, constipation, encopresis and refusal to go to the toilet more frequently. Therefore advice on the manners for toilet training is the most important consideration in treating constipation.
If the response to non-pharmacological measures is inadequate or difficult for the patient to achieve, several laxatives may be needed. Osmotic laxatives are generally prescribed as first line constipation treatment (Table 2) [41]. For patients with constipation who are being treated with an antimuscarinic for OAB symptoms, careful attention is needed not to cause or worsen constipation. If constipation develops due to antimuscarinic drug, dose adjustment or use of a different antimuscarinic are strongly recommended [42].
Conclusions
Several studies have shown that OAB is closely associated with constipation. The pathophysiology related with fecal retention supports this association. Therefore, when diagnosing OAB, fecal retention should always be considered [1-3]. However, the assessment of fecal retention during childhood may be difficult due to unreliable history or embarrassment of the child. Objective tools, such as scoring systems of plain abdominal radiography and the Bristol Stool Form Scale, can be helpful in estimating fecal retention [33, 34, 43]. For managing coexisting fecal retention and OAB, toilet training should be included in the treatment plan. Several osmotic laxatives are also effective in improving OAB symptoms and fecal retention. Additionally, bladder and bowel dysfunctions in children may continue into adulthood [44]. Therefore, adequate management of voiding and/or bowel dysfunction in children is of considerable importance.
Notes
Conflict of interest
Authors have declared that no competing interests exist.


