The Serum Level of Insulin Growth Factor-1 and Insulin Growth Factor Binding Protein-3 in Children with Henoch-Schönlein Purpura
Article information
Abstract
Purpose:
We investigated whether serum levels of insulin growth factor-1 (IGF-1) and insulin growth factor binding protein-3 (IGFBP-3) are valuable in predicting clinical outcomes or are correlated with other laboratory findings in children with Henoch-Schönlein purpura (HSP).
Methods:
We examined 27 children who were consecutively admitted to our hospital with HSP between January 2011 and February 2012. Blood tests (C-reactive protein, white blood cell count, platelet count, erythrocyte sedimentation rate, albumin, immunoglobulin A, complement C3, antineutrophil cytoplasmic antibody, IGF-1, IGFBP-3) and urine tests were performed upon admission. IGF-1 and IGFBP-3 were resampled in the recovery phase. Controls included 473 children whose IGF-1 and IGFBP-3 were sampled for evaluating their growth, at the outpatient department of pediatric endocrinology in our hospital. IGF-1 and IGFBP-3 were compared between the HSP children and controls, and between the acute and recovery phases in HSP children. The ability of these values to predict clinical outcomes including renal involvement was analyzed using bivariate logistic regression analysis (BLRA).
Results:
IGF-1 and IGFBP-3 were not different between the HSP children and controls (148.7±117.6 vs. 69.2±96.9, P =0.290: 3465.9±1290.9 vs. 3597.2±1,127.6, P =0.560, respectively). There was no significant difference in IGF-1 or IGFBP-3 between acute and recovery phases. Based on the BLRA, no variable, including IGF-1 and IGFBP-3, could predict clinical outcomes including the presence of nephritis
Conclusion:
We concluded that IGF-1 and IGFBP-3 do not predict clinical outcomes of HSP, including renal involvement, in this study.
Introduction
The long term prognosis of Henoch-Schönlein purpura (HSP) is determined by the degree of renal involvement [1].
Yildiz B et al. [2] reported in their study including 44 HSP patients published at 2008 that serum levels of Insulin growth factor-1 (IGF-1) and insulin growth factor binding protein-3 (IGFBP-3) were significantly higher in HSP than in the controls. Serum IGF-1 levels were significantly higher in HSP with proteinuria than those without proteinuria and controls. Serum immunoglobulin A (IgA)-complement C3 (C3) ratio was higher in HSP than in the controls. They insisted that IGF-1 and IGFBP-3 levels could be new markers for determination of renal involvement in HSP.
Ru L et al. [3] reported in their study including 31 HSP patients published at 2013 that HSP patients with higher pathological grades and HSP nephritis (HSPN) patients with proteinuria had higher serum levels of IGF-1 and IGFBP-3. Levels of white blood cells (WBC), red blood cells, platelet count, C3, IgG, and IgA and IgA-C3 ratio were significantly higher in the HSP and HSPN groups than in the control group. They also insisted that serum levels of IGF-1 and IGFBP-3 might be indicators of renal involvement in HSP patients.
In the present study, we aimed to investigate whether serum levels of IGF-1 and IGFBP-3 have values worthy of predicting clinical outcomes or are correlated with other laboratory findings in HSP children.
Materials and methods
1. Patients
This study included 27 children who were consecutively admitted to our hospital with HSP between January 2011 and Febrary 2012. The diagnostic criteria of HSP were as follows: palpable purpura (mandatory criterion) in the presence of at least one of the following four features: 1) diffuse abdominal pain, 2) any biopsy showing predominant IgA deposition, 3) arthritis or arthralgia (acute, any joint), and 4) renal involvement (any hematuria and/or proteinuria). The criteria of admission were as follow: severe abdominal pain, gastrointestinal bleeding, arthalgia with limitation of joint motion and joint swelling, or painful scrotal swelling. Intraveous methylprednisolone (1-2 mg/kg devided by three times) was administered on admission. Once one or two days after their critical symptoms were resolved, they discharged with oral prednisolone (solondo 1-1.5 mg/kg devided by three times), which was tapered off over 2-3 weeks. The dates of follow-up visit were one week, one month, and three months after discharge on schedule and urinalysis was done at every visit. Nephritis was defined by the presence of persistent microscopic hematuria at least more than three times, gross hematuria or association with proteinuria (urine protein to creatinine ratio >0.2).
Laboratory tests at admission included complete blood cell counts, C-reactive protein (CRP), erythrocyte sedimentation rate (ESR), serum albumin, blood urea nitrogen, serum creatinine, C3, serum IgA, antinuclear antibody, IGF-1, IGFBP-3, and urine analyses. At recovery phase when patients revisited after discharge, IGF-1 and IGFBP-3 were resampled. Written informed consent was obtained from parents of enrolled patients prior to blood tests. The CHA University Institutional Review Board approved this study including the consent procedure (CHA IRB No. BD 2011-136D).
Controls included 473 children among 581 children who sampled IGF-1 and IGFBP-3 for the evaluation of their growth at the outpatient department of pediatric endocrinology in our hospital from January 2008 to october 2015. One hundred eight of 581 children were excluded in order to match the mean age and sex ratio of controls with HSP patients. Their data were collected retrospectively.
All blood and urine data of HSP children were collected prospectively. The level of IGF-1 and IGFBP-3 were compared between HSP children and controls, and between acute and recovery phase in HSP children. Also, we investigated whether their levels were correlated with clinical presentations and those severity, and they could predict their clinical outcomes including nephritis.
2. Statistical analysis
All variables were presented as the mean±standard deviation., and continuous variables were analyzed using the student’s t-test. Qualitative variables and correlations were analyzed using the Pearson chi-squared test and Pearson correlation coefficient (two-tailed probability), respectively. Non-parametric variables were analyzed using Spearman’s rank correlation coefficient. To investigate the ability of IGF-1 and IGFBP-3 to predict a clinical outcome in children with HSP, bivariate logistic regression analysis (BLRA) was used. Statistical analysis was performed using SPSS statistics 20 (SPSS Inc, Chicago, IL). Statistical significance was defined as P≤0.05.
Results
The demographic characteristics of children with HSP were shown in Table 1. 14 HSP children (51.9%) had preceding upper respiratory infection (13) or acute gastroenteritis (1) mean 5.9±4.8 days before admission. All HSP patients had purpura on lower extremities for mean 3.4±2.8 days before admission. Purpura was presented on trunk (18.5%) and upper extremities (22.2%). Purpura was persisted during mean 3.5±5.4 weeks. Abdominal pain was presented in 48% of HSP patients, arthritis in 62.9%, and scrotal swelling in 7.4%. 29.6% of HSP children had both abdominal pain and arthritis at admission. Hematemesis and hematochezia were present in 1 and 4 patients, respectively. Multiple joint involvement was present in 18.5% of patients. Gross hematuria was not detected in this study population. Microscopic hematuria was detected in 8 patients (29.6%) at admission, of whom 7 patients had persistent microscopic hematuria and 2 patients had mild proteinuria (urine protein to creatinine ratio 0.67, 0.91, respectively). Enalapril (0.15 mg/kg) were administered in 2 patients with proteinuria for three months. Proteinuria free state was achieved one month after treatment. The mean period of admission was 4.9±3.2 days. Two patients experienced the recurrence of purpura over one month after the first onset. There was no additional admission due to recurrence of HSP in this study population within observational period (mean follow-up period, 10.9 months).
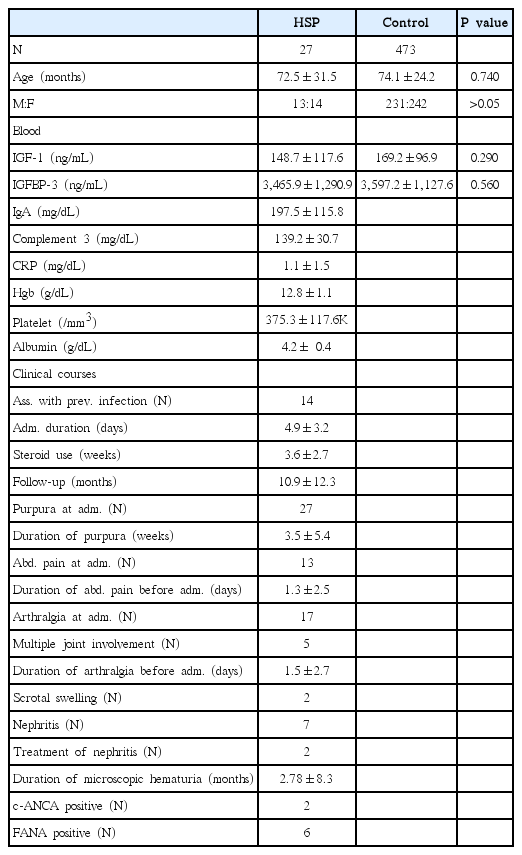
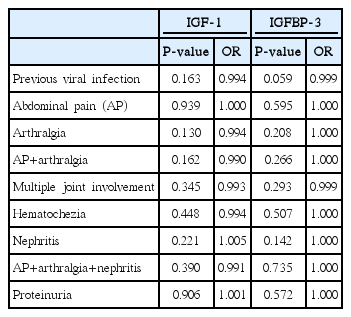
Clinical Courses of Children with Henoch-Schölein Purpura (HSP), and Comparison of Insulin Growth factor-1 (IGF-1) and Insulin Growth Factor Binding Protein-3 (IGFBP-3) between Children with HSP and Controls
There was no difference in the mean age and sex ratio between HSP children and controls (72.5±31.5 months vs. 74.1±24.2 months, P=0.740). Serum IGF-1 and IGFBP-3 were not different between two groups (148.7±117.6 vs. 169.2±96.9, P=0.290; 3,465.9.±1,315.5 vs. 3,597.2±1,127.6, P=0.160, respectively) (Table 1). Each serum IGF-1 and IGFBP-3 level in 13 HSP patients with abdominal pain, 17 HSP patients with arthritis, or 7 HSP patients with nephritis at admission were not different compared with controls (P=0.840, P=0.754, P=0.556, respectively). In this study population, low C3 level (<90 mg/dL), thrombocytopenia (<150,000/mm3), low serum hemoglobin (<10 g/dL), or low albumin level (< 3.5 g/dL) was not detected (Table 1).
Age of HSP children was positively correlated with the duration of steroid use and serum IgA (P=0.021, 0.020, respectively), while negatively correlated with the presence of hematochezia, positive stool occult blood (OB), and the presence of abdominal pain (AP) and arthralgia at visit (P=0.037, 0.009, 0.034, respectively). The duration of admission was positively correlated with the hematochezia, positive stool OB, and multiple joint involvement at visit (P=0.013, 0.011, 0.001, respectively), while negatively correlated with C3 and serum albumin (P=0.046, 0.009, respectively). The duration of AP was positively correlated with hematochezia (P=0.030). The use of steroid over 1 months was positively correlated with the presence of proteinuria (P=0.039), while negatively correlated with serum albumin (P=0.018). The duration of purpura before admission was positively correlated with the presence of proteinuria, C3, and serum WBC count (P=<0.001, 0.002, 0.043, respectively). Hematochezia was negatively correlated with C3 (P=0.024). The presence of proteinuria was positively correlated with the duration of purpura before admission, the duration of AP before admission, the duration of arthralgia before admission, total period of arthralgia, and the use of steroid over 1 month (P=<0.001, 0.023, <0.001, 0.012, 0.039, respectively), while negatively correlated with serum albumin (P=0.010). The presence of nephritis at visit was positively correlated with the duration of arthalgia before admission and hemoglobin level (P=0.015, 0.024, respectively). Serum albumin was negatively correlated with the duration of arthalgia before admission and total period of arthralgia (P=0.005, 0.007, respectively). WBC count was positively correlated with the duration of purpura before admission and multiple joint involvement (P=0.040, 0.036, respectively). IgA was positively correlated with the duration of purpura before admission, the presence of proteinuria, total period of nephritis, and ESR (P=0.002, 0.031, 0.032, 0.029, respectively). C3 was positively correlated with ESR (P=0.009). Abnormal anticoagulation test was positively correlated with CRP and WBC (P=0.021, 0.039, respectively). ESR was positively correlated with C3 and CRP (P=0.009, <0.001, respectively), while negatively with hemoglobin level (P=0.011).
Serum IGF-1 was positively correlated with IGFBP-3, serum hemoglobin, age, total period of persistent purpura, total duration of steroid use, and total duration of nephritis (P=0.000, 0.001, 0.000, 0.033, 0.011, 0.006, respectively), and negatively correlated with CRP and ESR (P=0.005, 0.021, respectively).
Serum IGFBP-3 was positively correlated with serum hemoglobin, serum albumin, age, and total duration of nephritis (P=0.000, 0.022, 0.000, 0.003, respectively), and negatively correlated with CRP (P=0.003).
Follow-up sampling of IGF-1 and IGFBP-3 was done in 8 HSP patients. Table 2 showed each of IGF-1 and IGFBP-3 was not significantly increased at the recovery phase of HSP (148.7±117.6 vs. 205.6±94.9, P=0.940; 3,465.9±1,315.5 vs. 4,516.3±916.9, P=0.120, respectively).
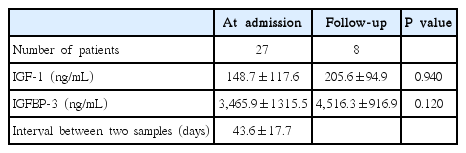
Follow-up of Insulin Growth Factor-1 (IGF-1) and Insulin Growth Factor Binding Protein-3 (IGFBP-3) in Henoch-Schönlein Purpura Children
By BLRA, no variable including IGF-1 and IGFBP-3 (Table 3) predicts clinical outcomes including the presence of nephritis except the following findings: preceding viral infection can predict the presence of both abdominal pain and arthralgia at visit (P=0.014); the total duration of abdominal pain, the period of abdominal pain before visit, stool occult blood, and serum IgA can predict the presence of proteinuria (P=0.040, 0.045, 0.015, 0.020, respectively). By multiple logistic regression analysis, no variable can predict the presence of proteinuria.
Discussion
HSP is a common multisystemic type of vasculitis associated with IgA mediated immune reaction with yet incompletely understood pathogenesis. Vessel inflammation and endothelial injury caused by IgA mediated immune reaction characterized by excessive production of proinflammatory cytokines, including interleukin (IL)-1, IL-6, IL-8, and tumor necrosis factor (TNF)-α, are possibly involved in HSP pathogenesis as demonstrated in the etiology of HSP [4,5]. Renal involvement is the principal cause of morbidity and mortality in children with HSP [6]. The proportion of HSP nephritis (HSPN) is at least more than 20% [7]. The renal manifestations can occur at any time, particularly within 1 month after the development of HSP [8]. The most common urinary abnormalities are albuminuria and microscopic hematuria. A smaller number of patients have macroscopic hematuria. Acute nephritic syndrome occurs in the more severe cases and may lead to nephrotic syndrome or renal insufficiency [9]. Kawasaki et al. [9]. reported that nephrotic syndrome, decreased factor XIII activity, hypertension and renal failure at onset belonged to clinically unfavorable prognostic factors in HSPN. Pathologically unfavorable factors in HSPN involved the rate of glomeruli with crescents, macrophage infiltrations, tubulointerstitial changes and acute exacerbation. In addition, they reported the expression of renal alpha-smooth muscle actin that is the predominant actin isoform within vascular smooth muscle cells and plays an important role in fibrogenesis was a predictor of poor prognosis in HSPN patients [9]. Shin et al. [10]. reported serum IgA/C3 ratio may be a useful marker of disease activity in severe Henoch-Schönlein nephritis.
There have been no other known predictor of the poor prognosis as well as the development of HSPN in HSP patients, except IGF-1, IGFBP-3, and genetic susceptibility reported in a few articles on the literature search. In this study, by BLRA as well as correlation coefficient, the duration of AP before visit and serum IgA were significant predictors of the presence of proteinuria in HSP children.
IGF-1 is a polypeptide growth factor that plays a significant role in cellular growth and survival, regarding pathological states in particular [2]. IGF-1 enhances TNF-α and TNF-α induced expression of adhesion molecules in endothelial cells. Besides, IGF-1 may stimulate angiogenesis, fibrosis and tubular formation in endothelial cells and IGF-1 increases glomerular filtration rate [2]. However, the role of IGF-1 in HSP is unclear. IGF-1 is an enhancing factor for cytokine-induced endothelial cell inflammation in HSP vasculitis [11]. Increased IGF-1 and IGFBP-3 in HSP patients might protect kidney from glomerular crescents through those renal vascular effects which causes activation of two endogenous vasodilators, nitric oxide and prostaglandins [12]. IGF-1 had fibrotic effects through increasing collagen and fibronectin protein, enhancing collagen transcription [13]. On the contrary, IGF-1 has been suggested to reduce fibrosis, while its ability to inhibit apoptosis or vasodilate blood vessels could also inhibit the fibrotic process [14]. Yildiz B et al. [2]. reported in their study that IGF-1 and IGFBP-3 levels increased in the acute onset of HSP, and these increased levels could be related to degree of the proteinuria. Therefore, IGF-1 and IGFBP-3 levels may be indicative of a renal involvement.
Although the actual cause of the endothelial damage in HSP is not well known, increasing evidence suggests that the activated neutrophils injure endothelial cells through the generation of reactive oxygen species which has been implicated as likely candidates [15]. Chen T et al. [16]. reported that elevated heme oxygenases was correlated with the overall clinical score in the active phase of HSP patients. Also, oxidative stress may be associated with the elevation of heme oxygenases level in HSP. Furthermore, their study including 36 patients with different phases of HSP showed that IGF-1 levels in active phase and early resolution phase of HSP were significantly higher than those in normal controls. IGF-1 level was significantly higher in patients with internal organ involvement than in those without internal organ involvement. They presumed that the elevated IGF-1 level in HSP may be beneficial to protect the endothelial damages induced by oxidative stresses in HSP.
However, in this study, either IGF-1 or IGFBP-3 could not be a relavant factor to predict any clinical outcome including proteiuria or nephritis. Furthemore, the increase of these levels at recovery phase in HSP children was not significant. Therefore, IGF-1 and IGFBP-3 were not useful markers to predict clinical outcomes including renal involvement in HSP children.
Recently, Ge W et al. [17] reported that plasma pentraxin 3 could be a potential predictor of HSPN in HSP children (sensitivity 73.0%, specificity 79.6%). Pentraxin 3 is the prototype of long pentraxins produced by various cells in peripheral tissues, including vascular endothelial cells, macrophages, dendritic cells, fibroblasts, myeloid cells, and smooth muscle cells in response to pro-inflammatory cytokines and endotoxins, such as lipopolysaccharide, IL-1, and TNF-α. But, its sensitivity and specificity was so low that we can not consider it as a useful marker of renal prognosis in HSP children.
The present study has the following limitations: (1) the size of study population was small; (2) the severity of HSP nephritis in this study was milder than in other related studies; (3) the data of contol were collected retrospectively, and (4) there were no data of comparative laboratory findings in controls except IGF-1 and IGFBP-3.
Conclusion
In conclusion, IGF-1 and IGFBP-3 levels do not predict clinical outcomes of HSP, including renal involvement in this study.