Efficacy and safety of losartan in childhood immunoglobulin A nephropathy: a prospective multicenter study
Article information
Abstract
Purpose
Angiotensin-converting enzyme inhibitors and angiotensin II receptor blockers (ARBs) are frequently employed to counteract the detrimental effects of proteinuria on glomerular diseases. However, the effects of ARBs remain poorly examined in pediatric patients with immunoglobulin A (IgA) nephropathy. Herein, we evaluated the efficacy and safety of losartan, an ARB, in pediatric IgA nephropathy with proteinuria.
Methods
This prospective, single-arm, multicenter study included children with IgA nephropathy exhibiting proteinuria. Changes in proteinuria, blood pressure, and kidney function were prospectively evaluated before and 4 and 24 weeks after losartan administration. The primary endpoint was the difference in proteinuria between baseline and 24 weeks.
Results
In total, 29 patients were enrolled and received losartan treatment. The full analysis set included 28 patients who received losartan at least once and had pre- and post-urinary protein to creatinine ratio measurements (n=28). The per-protocol analysis group included 22 patients who completed all scheduled visits without any serious violations during the study period. In both groups, the mean log (urine protein to creatinine ratio) value decreased significantly at 6 months. After 24 weeks, the urinary protein to creatinine ratio decreased by more than 50% in approximately 40% of the patients. The glomerular filtration rate was not significantly altered during the observation period.
Conclusions
Losartan decreased proteinuria without decreasing kidney function in patients with IgA nephropathy over 24 weeks. Losartan could be safely employed to reduce proteinuria in this patient population. ClinicalTrials.gov trial registration (NCT0223277)
Introduction
Immunoglobulin A (IgA) nephropathy is the most common glomerular disease and is often diagnosed in children and young adults. Given that a considerable proportion of IgA nephropathy progresses to chronic kidney disease (CKD), especially when proteinuria is ≥1 g/day, the therapeutic goal is to reduce proteinuria. Blockage of the renin-angiotensin system (RAS) to reduce proteinuria has been the mainstay of IgA nephropathy treatment [1-4], along with various immunosuppressive medications [5-7].
The RAS is a hormonal system that plays a crucial role in blood volume and systemic vascular resistance. Activation of RAS results in increased blood pressure and sodium and water retention, subsequently increasing the effective circulating volume. RAS blockade reduces systemic and intraglomerular hydrostatic pressures by inhibiting angiotensin II-mediated efferent arteriolar vasoconstriction. Renal angiotensin II plays a major role in the development of renal fibrosis that progresses to end-stage renal failure, and the degree of proteinuria is known to be a risk factor for progression to kidney failure [8-10]. The ability of angiotensin II receptor blockers (ARBs) to reduce proteinuria, thereby delaying CKD progression, has been well-examined in diabetic nephropathy with proteinuria [11], leading to the approval of losartan and irbesartan for this indication. In IgA nephropathy, ARBs are frequently employed in combination with other therapeutic agents [12,13], and in adults, low-dose losartan was proven to reduce proteinuria [14]. Also, the blockade of RAS alone appears to be effective in children with proteinuria induced by kidney disease [15]. Among ARB, losartan has been approved by the U.S. Food and Drug Administration (FDA) for delaying CKD progression in proteinuric diabetic nephropathy along with irbesartan, although only losartan has been approved for children >5 years of age.
Angiotensin-converting enzyme inhibitors (ACEIs) are often selected as the first choice of RAS blocking agent; however, some patients fail to tolerate ACEIs and are switched to ARBs. A high incidence of ACEI-induced side effects such as dry cough has been documented in East Asia [16]. Therefore, ARBs have a relatively high clinical significance in this region. However, the use of ARBs for proteinuric glomerulopathy other than diabetic nephropathy remains off-label. In addition, the efficacy of ARB alone remains unexplored among pediatric patients with IgA nephropathy. In the present study, we performed a prospective multicenter study to confirm the efficacy and safety of losartan in reducing proteinuria in normotensive childhood IgA nephropathy with persistent proteinuria in Korea.
Methods
Subjects
Herein, children aged 24 months to 18 years who were diagnosed with IgA nephropathy based on kidney biopsies performed at participating hospitals were enrolled if they fulfilled the following two conditions: (1) estimated glomerular filtration rate (eGFR) >60 mL/min/1.73 m2, and (2) a mean urine protein to creatinine ratio (UPCR) in the first morning urine sample of 3 consecutive days of ≥0.3. Patients with the following conditions were excluded: hypertension, allograft kidney transplantation, renal artery stenosis, confirmed primary hyperaldosteronism, history of hypersensitivity reactions to ARB, pregnancy, lactation, treatment with immunosuppressive agents such as steroids or calcineurin inhibitors, or taking antihypertensive drugs other than ACEIs or ARBs for blood pressure regulation. The number of subjects required to assess the efficacy of losartan in reducing proteinuria was calculated to be 28, assuming the following: level of significance α=0.05, type 2 error β=0.10, power of the test 80%, drop-out rate 10%, and magnitude of protein reduction 35.8%, according to the study by Webb et al. [17].
Methods
Study design
This study was approved by the Korean FDA and Institutional Review Boards of all participating institutions and conducted according to the Declaration of Helsinki. The trial was registered with ClinicalTrials.gov under the trial registration number NCT02232776. As a placebo-controlled study was assumed to be unethical, this study was designed as a single-arm study. The enrollment of study subjects was conducted from September 2014 to October 2016, with participation from seven centers in Korea. Patients who agreed to participate in the study were screened after obtaining written consent from the subjects and their legal representatives and enrolled if they met the pre-determined inclusion and exclusion criteria. At 2, 4, 12, and 24 weeks after initiating losartan, patients were assessed for proteinuria in the first morning urine, blood pressure, and potential side effects. Complete blood count and serum chemistry tests were performed at 12 and 24 weeks. Blood pressure was measured on two occasions, with a 1- to 3-minute interval between measurements, using the oscillometric method. The mean value obtained from these measurements was used for analysis. Losartan was initiated at a dose of 0.7 mg/kg/day, taken once daily. At the 2- and 4-week follow-ups, the losartan dose was increased if proteinuria persisted given the absence of side effects, up to a maximum dose of 1.5 mg/kg/day or 100 mg/day. Complete remission was defined as a UPCR of <0.2, and partial remission was defined as a UPCR reduction of >50%. Proteinuria was evaluated using the first-morning UPCR. The eGFR was calculated using the bedside Chronic Kidney Disease in Children (CKiD) formula.
The primary endpoint was the change in proteinuria at 6 months after treatment. The secondary endpoints were as follows: (1) changes in proteinuria 1 month after dosing, (2) complete remission rate at 6 months, (3) partial remission rate percentage 6 months after administration, and (4) changes in eGFR at 6 months after dosing.
Statistical analysis
Subjects who had taken losartan at least once and had pre- and post-UPCR were grouped into the full analysis set (FAS) group. Subjects who completed all scheduled visits without any serious violations were grouped into the per-protocol (PP) group. Patients were excluded from the PP group if they had (1) hypertension requiring additional antihypertensive drugs or hypotension necessitating ARB discontinuation, (2) an eGFR of <60 mL/min/1.73 m2, and (3) non-compliance with the visit schedule. For statistical analysis, the FAS group was used as the main analysis group and the PP group as the auxiliary group. Primary and secondary endpoints and adverse events, such as kidney dysfunction, hyperkalemia, angioedema, and changes in blood pressure, were analyzed. The UPCR was analyzed using a natural logarithm to satisfy the assumption of normality. The paired t-test was used when the difference in the log UPCR at the 6-month follow-up before and after the medication satisfied the assumption of normality, and the Wilcoxon signed-rank test was employed when the assumption of normality was not satisfied. Categorical data are presented as frequencies and percentages, and 95% confidence interval (CIs) were estimated. Continuous data are presented as mean±standard deviation or median (interquartile range [IQR]).
Results
Patients’ characteristics
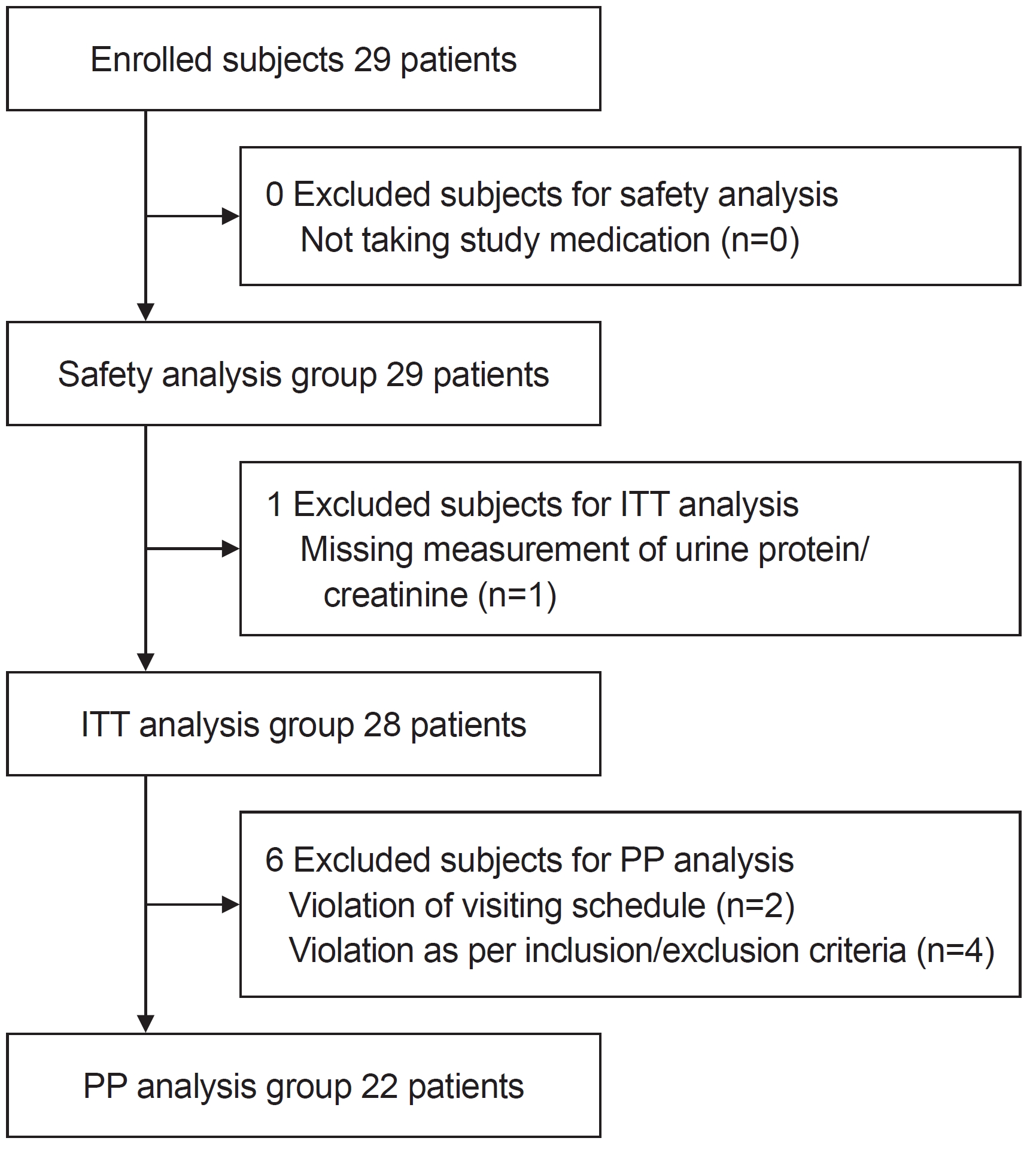
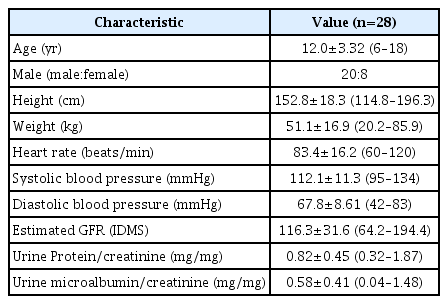
In this prospective study, 29 subjects with biopsy-proven IgA nephropathy were enrolled and received losartan treatment at the median age of 12.2 years (IQR, 10.1–15.0 years). Among them, the UPCR was not assessed in one patient after receiving losartan, who was excluded from the FAS group (n=28). This group is defined as the intent-to-treat (ITT) population (Fig. 1). In the ITT group, three patients had hypertension before losartan administration, one patient exhibited a decreased eGFR <60 mL/min/1.73 m2 at the 2-week visit after initiating losartan, and two did not complete the visit schedule; therefore, they were excluded from the PP group (n=22). Table 1 summarizes the basic clinical characteristics of the ITT group. The median disease duration was 3.12 years (IQR, 0.51–4.42 years). At enrollment, the median baseline proteinuria was 0.72 mg/mg (IQR, 0.48–0.95 mg/mg) in the FAS group.
Outcomes
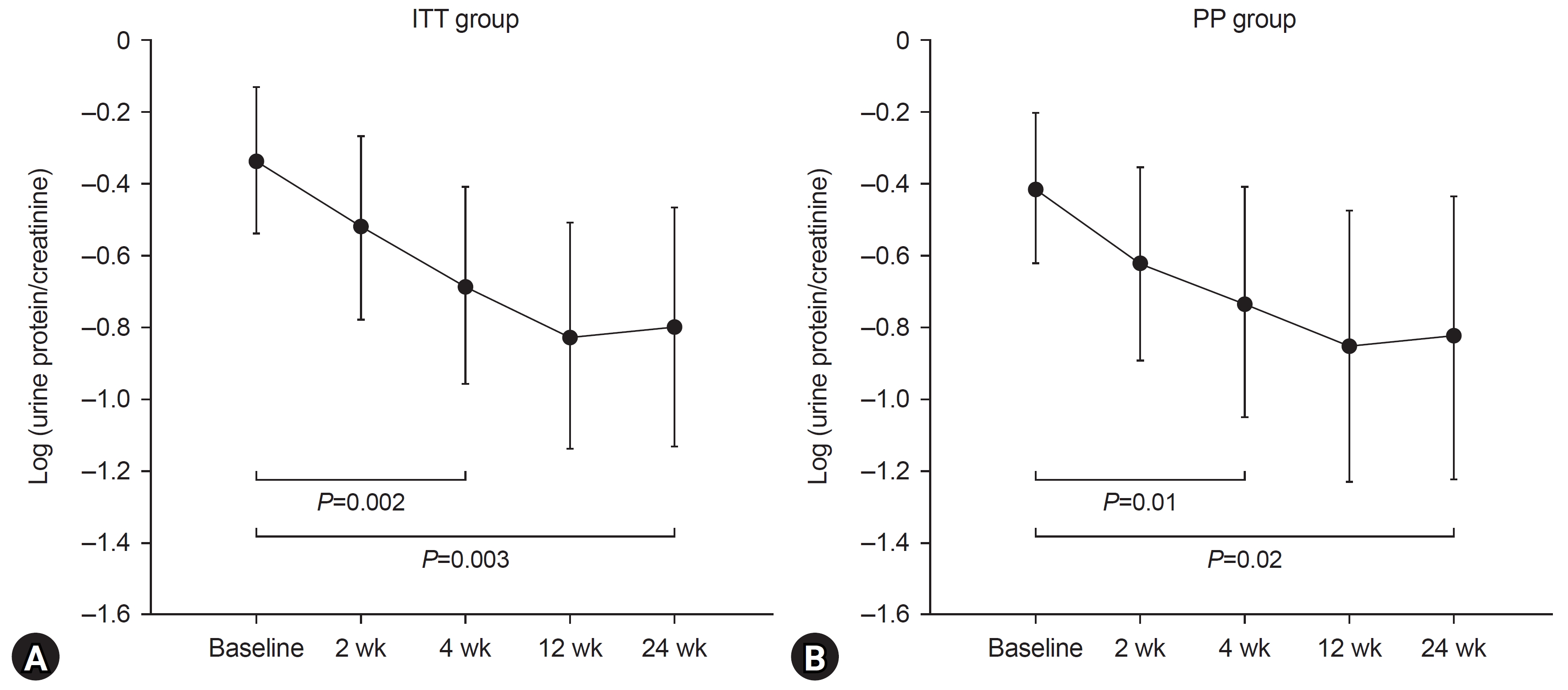
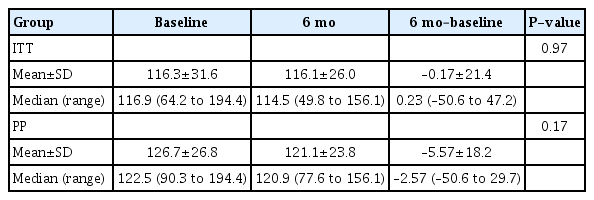
In the ITT group, the mean log UPCR value before losartan administration was –0.34, which significantly decreased to –0.80 at 6 months of administration (P=0.003). In the PP group, we noted a significant decrease in the mean log UPCR value at 6 months of administration (P=0.02) (Fig. 2). In both groups, the mean log UPCR was significantly reduced 1 month after administration when compared with that before administration (Fig. 2). Following 6 months of treatment, proteinuria disappeared (UPCR was less than 0.2) in 14.3% (95% CI, 4.02%–32.7%) and 13.6% (95% CI, 2.90%–34.9%) of patients in the ITT and PP groups, respectively. Six months after treatment, a >50% reduction in UPCR was observed in 39.3% (95% CI, 21.2%–57.4%) and 36.7% (95% CI, 16.3%–56.5%) of the FAS and PP groups, respectively. The eGFR values before and after losartan administration did not differ significantly (Table 2).
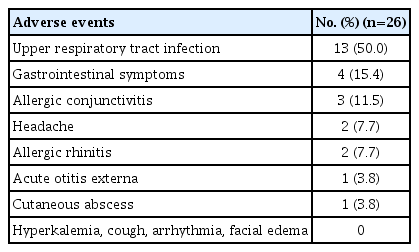
Safety
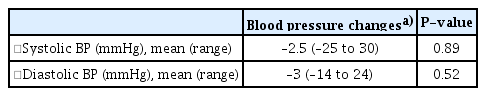
During the study period, nine (31.0%) of the 29 subjects who received losartan treatment experienced one or more adverse events. Overall, 26 adverse events were reported, of which one was a significant adverse reaction involving a cutaneous abscess that occurred in a 12-year-old male subject; however, there was no causal relationship with the drug (Table 3). No hyperkalemia or angioedema was observed during the study period. Following 6 months of losartan treatment, the median values for altered systolic and diastolic blood pressure were –2.50 mmHg (IQR, –5.50 to 9.00 mmHg) and –3.0 mmHg (IQR, –7.00 to 4.50 mmHg), respectively. There was a slight decrease in blood pressure, although symptoms such as dizziness were not observed (Table 4).
Discussion
The ESCAPE (Effect of Strict Blood Pressure Control and ACE Inhibition on Progression of Chronic Renal Failure in Pediatric Patients) study and that by Webb et al. have confirmed the efficacy of RAS blockade for reducing proteinuria in children [17,18]. The ESCAPE study has revealed that strict blood pressure control can effectively delay the progression of CKD with proteinuria in children receiving ACEIs [18]. However, in the ESCAPE study, similar to several other studies conducted in adults, the efficacy of RAS inhibition for suppressing proteinuria was not examined independently from the effect of blood pressure reduction mediated by RAS inhibition on proteinuria. In addition, the underlying diseases of the study participants were heterogeneous. In the present study, the efficacy of RAS inhibition on proteinuria in pediatric IgA nephropathy was evaluated independently of the antihypertensive effect of losartan. According to the study by Webb et al. [17], losartan could effectively reduce proteinuria in pediatric patients with CKD when compared with a calcium channel blocker, amlodipine or placebo. Although amlodipine and placebo increased proteinuria by 1.4%, losartan reduced proteinuria by 35.8%. In addition, several prospective studies have evaluated the efficacy and safety of ACEIs with or without ARB in children [19-21]. A randomized controlled trial assessing pediatric patients with IgA nephropathy in Japan has supported lisinopril monotherapy in reducing proteinuria when compared with combination therapy with lisinopril and losartan. The cumulative disappearance rate of proteinuria at 24 months was 89.3% with combination therapy and 89% for monotherapy, without differences in safety [19]. Conversely, the long-term efficacy of losartan and enalapril treatment revealed that the reduction in proteinuria at 3 years was 30.0% for losartan and 40.5% for enalapril [20]. These studies have shown that either ACEI or ARB therapy can be effective in pediatric patients with IgA nephropathy.
In the present study, we aimed to evaluate the efficacy and safety of losartan in children with IgA nephropathy and provide medical evidence for the use of losartan in children with proteinuric kidney disease in Korea. Losartan is an angiotensin II type I receptor blocker that blocks the angiotensin system, inhibits kidney damage, and reduces urinary proteinuria. To the best of our knowledge, this is the first prospective clinical study to examine the potential of losartan monotherapy in children with IgA nephropathy. Our study supports the finding that losartan could effectively reduce proteinuria in pediatric patients with IgA nephropathy.
IgA nephropathy is known to exhibit a relatively benign clinical course in children when compared with that in adults [22-25]. In a Japanese study, although adult IgA nephropathy was found to progress to kidney failure in 10%–20% and 50% of patients within 10 and 20 years, respectively, kidney survival rates at 10- and 20-year were 95% and 82%, respectively, in children [23]. In a retrospective study assessing 99 pediatric patients with IgA nephropathy and 125 adult patients with IgA nephropathy, pediatric patients were more likely to display minimal histologic lesions, and 10-year kidney survival was 87% in children when compared with 48% in adults [26]. In children, the probability of spontaneous remission without treatment was found to be higher than the predicted probability [27]. Most subjects in this study also showed improvements in proteinuria with losartan treatment.
A recent study from Korea has shown that 65 (5.6%) of 1,154 pediatric patients with IgA nephropathy developed CKD stage 3-5 during a median follow-up of 5 years. In total, 947 patients were treated with RAS blockers with or without additional immunosuppressive drugs, and 207 patients (17.9%) received no pharmacologic treatments. Considering proteinuria, the authors found that 839 patients (72.7%) achieved remission. Moreover, remission was found to be an independent prognostic factor that negatively correlated with the progression of CKD stage 3-5 (hazard ratio, 0.19; 95% CI, 0.06–0.26; P<0.001) [28]. Accordingly, these findings highlight that the prognosis of children with IgA nephropathy in Korea is relatively good, and achieving remission indicates a favorable outcome of IgA nephropathy.
Few studies have established that losartan can be safely used in children and adolescents [18,29]. Dizziness or headache is common due to drug-related adverse reactions, which can be reversed by appropriate dose adjustment, and sometimes due to side effects, in which case the medication may be withdrawn for a short period and subsequently re-initiated [18]. Compared with ACEIs, losartan can be safely employed in terms of potential side effects [20,30]. However, when combined with tacrolimus, careful attention should be paid to hyperkalemia and metabolic acidosis. In addition, hyperkalemia may occur when losartan is combined with ACEI in CKD, and potassium concentration should be observed [21,31]. Herein, we observed no adverse events, such as decreased blood pressure, reduced GRF, or hyperkalemia, during the 24-week study period. There were no instances of relatively common side effects such as angioedema or hyperkalemia, which may be attributed to the limited number of subjects in the study. In the present study, losartan was initiated at a dose of 0.7 mg/kg/day, once daily, and was gradually increased (12.5–25.0 mg/day) up to a maximum dose of 1.5 mg/kg/day or 100 mg/day. For children, doses were initially estimated using adult doses, and the dose of losartan used in the present study is known to be safe for children and adolescents [17,19,20,32]. Considering children ˂6 years of age undergoing treatment for hypertension, doses of 0.1 to 0.7 mg/kg are effective, and doses of 1.4 mg/kg can be used without difficulty [32].
The present study was the first prospective, multicenter study conducted in Korea. However, its limitations need to be stated, including the relatively short study period, a small number of study subjects, and a single-arm design. An additional limitation of this study lies in the absence of consideration of histopathological findings for IgA nephropathy. The inclusion criteria encompassed children between 24 months and 18 years of age. However, since all enrolled participants were 6 years or older, the assessment of efficacy and safety in patients below this age threshold is limited
In conclusion, this study represents the first multicenter study for the use of losartan in pediatric patients with IgA nephropathy within our country. The results of this study demonstrate that losartan treatment led to a substantial reduction in proteinuria among children with IgA nephropathy, all while exhibiting a favorable safety profile devoid of severe adverse events. As such, these findings support the effective and safe application of losartan in managing IgA nephropathy in pediatric populations.
Notes
Ethical statements
This study was approved by the Korean FDA and Institutional Review Boards of all participating institutions including Seoul National University (IRB No. H1407113596), and conducted according to the Declaration of Helsinki. Informed consent was obtained.
Conflicts of interest
Eujin Park, Eun Mi Yang, Jin Soon Suh, Jae Il Shin, Hae Il Cheong, and Hee Gyung Kang are editorial board members of the journal but were not involved in the peer reviewer selection, evaluation, or decision process of this article. No other potential conflicts of interest relevant to this article were reported.
Funding
This research was supported by a grant (14172MFDS178) from the Ministry of Food and Drug Safety in 2014.
Author contributions
Conceptualization: HH, YHA, HGK, SHK
Data curation: EP, HJC
Formal analysis: KHH, JWL
Funding acquisition: HGK, ISH, HIC
Investigation: HJC, KHH, MHC, JWK, SYK, KHK, HWP
Methodology: JSS, JIS, MHC, HGK
Project administration: JWK, KHK
Visualization: HH, YHA
Writing–original draft: HH, YHA
Writing–review & editing: HGK, SHK
All authors read and approved the final manuscript.